The role of kelp species as biogenic habitat formers in coastal marine ecosystems.
J. Exp. Mar. Biol. Ecol. 2017; 492: 81-98
A seaweed aquaculture imperative to meet global sustainability targets.
Nat. Sustain. 2022; 5: 185-193
An overview of potential seaweed-derived bioactive compounds for pharmaceutical applications.
Mar. Drugs. 2022; 20: 141
Yearbook of Fishery and Aquaculture Statistics 2019.
FAO, 2021
Seaweed production: overview of the global state of exploitation, farming and emerging research activity.
Eur. J. Phycol. 2017; 52: 391-406
Kelp aquaculture in China: a retrospective and future prospects.
Rev. Aquac. 2021; 13: 1324-1351
Furthering knowledge of seaweed growth and development to facilitate sustainable aquaculture.
New Phytol. 2017; 216: 967-975
Seaweed cultivation: potential and challenges of crop domestication at an unprecedented pace.
New Phytol. 2015; 206: 489-492
Biorefinery of marine macroalgae into high-tech bioproducts: a review.
Environ. Chem. Lett. 2021; 19: 969-1000
An overview of marine macroalgae as bioresource.
Renew. Sust. Energ. Rev. 2018; 91: 165-179
Varying reproductive success under ocean warming and acidification across giant kelp (Macrocystis pyrifera) populations.
J. Exp. Mar. Biol. Ecol. 2020; 522151247
Future climate change is predicted to affect the microbiome and condition of habitat-forming kelp.
Proc. R. Soc. B Biol. Sci. 2019; 28620181887
Exposure to simulated heatwave scenarios causes long-term reductions in performance in Saccharina latissima.
Mar. Ecol. Prog. Ser. 2019; 630: 25-39
Climate change and disease: bleaching of a chemically defended seaweed.
Glob. Change Biol. 2011; 17: 2958-2970
Epiphytism, diseases and grazing in seaweed aquaculture: a comprehensive review.
Rev. Aquac. 2022; 14: 1345-1370
Impacts of ocean warming on kelp forest ecosystems.
New Phytol. 2020; 225: 1447-1454
Rise of turfs: a new battlefront for globally declining kelp forests.
BioScience. 2018; 68: 64-76
A community perspective on the concept of marine holobionts: current status, challenges, and future directions.
PeerJ. 2021; 9e10911
The seaweed holobiont: understanding seaweed–bacteria interactions.
FEMS Microbiol. Rev. 2013; 37: 462-476
Microbial dysbiosis: rethinking disease in marine ecosystems.
Front. Microbiol. 2016; 7: 991
Shaping the future of probiotics and prebiotics.
Trends Microbiol. 2021; 29: 667-685
Plant microbiome engineering: expected benefits for improved crop growth and resilience.
Trends Biotechnol. 2020; 38: 1385-1396
A global network meta-analysis of the promotion of crop growth, yield, and quality by bioeffectors.
Front. Plant Sci. 2022; 13816438
Improving crop yield and nutrient use efficiency via biofertilization – a global meta-analysis.
Front. Plant Sci. 2018; 8: 2204
From microbial dynamics to functionality in the rhizosphere: a systematic review of the opportunities with synthetic microbial communities.
Front. Plant Sci. 2021; 12650609
New frontiers in agriculture productivity: optimised microbial inoculants and in situ microbiome engineering.
Biotechnol. Adv. 2019; 37107371
Metabolic complementarity between a brown alga and associated cultivable bacteria provide indications of beneficial interactions.
Front. Mar. Sci. 2020; ()
Macroalgal-bacterial interactions: identification and role of thallusin in morphogenesis of the seaweed Ulva (Chlorophyta).
J. Exp. Bot. 2020; 71: 3340-3349
Microbiota influences morphology and reproduction of the brown alga Ectocarpus sp.
Front. Microbiol. 2016; 7: 197
The auxin-producing Bacillus thuringiensis RZ2MS9 promotes the growth and modifies the root architecture of tomato (Solanum lycopersicum cv. Micro-Tom).
Arch. Microbiol. 2021; 203: 3869-3882
Analysis of algal growth- and morphogenesis-promoting factors in an integrated multi-trophic aquaculture system for farming Ulva spp.
Aquac. Environ. Interact. 2019; 11: 375-391
Enhanced nitrogen and phosphorus activation with an optimized bacterial community by endophytic fungus Phomopsis liquidambari in paddy soil.
Microbiol. Res. 2019; 221: 50-59
From model organism to application: bacteria-induced growth and development of the green seaweed Ulva and the potential of microbe leveraging in algal aquaculture.
Semin. Cell Dev. Biol. 2022; ()
Conspecificity of the model organism Ulva mutabilis and Ulva compressa (Ulvophyceae, Chlorophyta).
J. Phycol. 2019; 55: 25-36
Engineering bacteria-seaweed symbioses for modulating the photosynthate content of Ulva (Chlorophyta): significant for the feedstock of bioethanol production.
Algal Res. 2020; 49101945
Isolation of Hyphomonas strains that induce normal morphogenesis in protoplasts of the marine red alga Pyropia yezoensis.
Microb. Ecol. 2014; 68: 556-566
Spore release in Acrochaetium sp. (Rhodophyta) is bacterially controlled.
J. Phycol. 2007; 43: 235-241
Seaweed-microbial interactions: key functions of seaweed-associated bacteria.
FEMS Microbiol. Ecol. 2014; 88: 213-230
Regulation of algal and cyanobacterial auxin production, physiology, and application in agriculture: an overview.
J. Appl. Phycol. 2021; 33: 2995-3023
Influence of phytohormones on morphology and chlorophyll a fluorescence parameters in embryos of Fucus vesiculosus L. (Phaeophyceae).
Russ. J. Plant Physiol. 2013; 60: 176-183
Role of bacterial isolates in enhancing the bud induction in the industrially important red alga Gracilaria dura.
FEMS Microbiol. Ecol. 2011; 76: 381-392
A mesocosm study on bacteria-kelp interactions: Importance of nitrogen availability and kelp genetics.
J. Phycol. 2021; 57: 1777-1791
‘Roots’ in mixotrophic algae.
Nature. 1996; 381: 382
How do microbiota associated with an invasive seaweed vary across scales?.
Mol. Ecol. 2020; 29: 2094-2108
Nitrogen fixation associated with the marine macroalga Codium fragile.
Limnol. Oceanogr. 1975; 20: 815-823
Draft genome and description of Waterburya agarophytonicola gen. nov. sp. nov. (Pleurocapsales, Cyanobacteria): a seaweed symbiont.
Antonie Van Leeuwenhoek. 2021; 114: 2189-2203
Rhodobacteraceae on the marine brown alga Fucus spiralis are abundant and show physiological adaptation to an epiphytic lifestyle.
Syst. Appl. Microbiol. 2017; 40: 370-382
Cross-exchange of B-vitamins underpins a mutualistic interaction between Ostreococcus tauri and Dinoroseobacter shibae.
ISME J. 2019; 13: 334-345
Ice-Ice disease: an environmentally and microbiologically driven syndrome in tropical seaweed aquaculture.
Rev. Aquac. 2022; 14: 414-439
Preliminary survey of pests and diseases of eucheumatoid seaweed farms in the Philippines.
J. Appl. Phycol. 2021; 33: 2391-2405
Pathogen-induced defense and innate immunity in macroalgae.
Biol. Bull. 2007; 213: 290-302
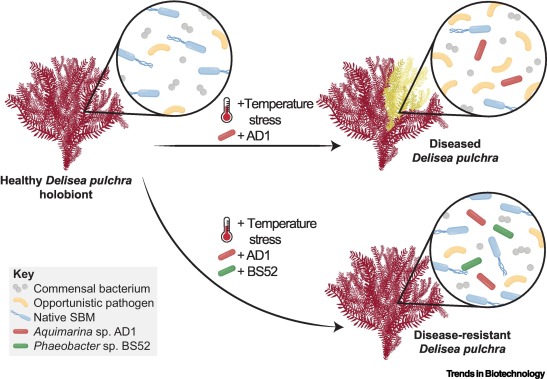
Bacterial controlled mitigation of dysbiosis in a seaweed disease.
ISME J. 2022; 16: 378-387
Cross-host protection of marine bacteria against macroalgal disease.
Microb. Ecol. 2021; ()
Microbial “gardening” by a seaweed holobiont: Surface metabolites attract protective and deter pathogenic epibacterial settlement.
J. Ecol. 2019; 107: 2255-2265
Chemical mediation of ternary interactions between marine holobionts and their environment as exemplified by the red alga Delisea pulchra.
J. Chem. Ecol. 2012; 38: 442-450
Temperature induced bacterial virulence and bleaching disease in a chemically defended marine macroalga.
Environ. Microbiol. 2011; 13: 529-537
Demographic consequences of disease in a habitat-forming seaweed and impacts on interactions between natural enemies.
Ecology. 2014; 95: 142-152
Multiple opportunistic pathogens can cause a bleaching disease in the red seaweed Delisea pulchra.
Environ. Microbiol. 2016; 18: 3962-3975
Genomes and virulence factors of novel bacterial pathogens causing bleaching disease in the marine red alga Delisea pulchra.
PLoS One. 2011; 6e27387
Microbial community function in the bleaching disease of the marine macroalgae Delisea pulchra.
Environ. Microbiol. 2017; 19: 3012-3024
A comprehensive analysis of the microbial communities of healthy and diseased marine macroalgae and the detection of known and potential bacterial pathogens.
Front. Microbiol. 2015; 6: 146
Community structure and functional gene profile of bacteria on healthy and diseased thalli of the red seaweed Delisea pulchra.
PLoS One. 2012; 7e50854
Interactions within the microbiome alter microbial interactions with host chemical defences and affect disease in a marine holobiont.
Sci. Rep. 2019; 9: 1363
Chemically-mediated interactions between macroalgae, their fungal endophytes, and Protistan pathogens.
Front. Microbiol. 2018; 9: 03161
Competitive interactions in mixed-species biofilms containing the marine bacterium Pseudoalteromonas tunicata.
Appl. Environ. Microbiol. 2005; 71: 1729-1736
Phylogenetic relationship and antifouling activity of bacterial epiphytes from the marine alga Ulva lactuca.
Environ. Microbiol. 2000; 2: 343-347
Competition for iron drives phytopathogen control by natural rhizosphere microbiomes.
Nat. Microbiol. 2020; 5: 1002-1010
Potential of a quorum quenching bacteria isolate Ochrobactrum intermedium D-2 against soft rot pathogen Pectobacterium carotovorum subsp. carotovorum.
Front. Microbiol. 2020; 11: 898
Can targeted defense elicitation improve seaweed aquaculture?.
J. Appl. Phycol. 2019; 31: 1845-1854
Plant immune system activation is necessary for efficient root colonization by auxin-secreting beneficial bacteria.
Cell Host Microbe. 2021; 29: 1507-1520
Origin and evolution of the plant immune system.
New Phytol. 2019; 222: 70-83
Indirect reduction of Ralstonia solanacearum via pathogen helper inhibition.
ISME J. 2022; 16: 868-875
A simplified synthetic community rescues Astragalus mongholicus from root rot disease by activating plant-induced systemic resistance.
Microbiome. 2021; 9: 217
Macroalgal–bacterial interactions: role of dimethylsulfoniopropionate in microbial gardening by Ulva (Chlorophyta).
Mol. Ecol. 2018; 27: 1808-1819
Salt-induced recruitment of specific root-associated bacterial consortium capable of enhancing plant adaptability to salt stress.
ISME J. 2021; 15: 2865-2882
Crying out for help with root exudates: adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes.
Curr. Opin. Microbiol. 2019; 49: 73-82
Chemically mediated microbial “gardening” capacity of a seaweed holobiont is dynamic.
Microorganisms. 2020; 8: 1893
Exploring the cultivable Ectocarpus microbiome.
Front. Microbiol. 2017; 8: 2456
Genome sequences of 72 bacterial strains isolated from Ectocarpus subulatus: a resource for algal microbiology.
Genome Biol. Evol. 2020; 12: 3647-3655
Bacterial responses to osmotic challenges.
J. Gen. Physiol. 2015; 145: 381-388
Seaweed resources in India – current status of diversity and cultivation: prospects and challenges.
Bot. Mar. 2019; 62: 463-482
Marine macroalgal nursery: a model for sustainable production of seedlings for large scale farming.
Algal Res. 2018; 31: 463-468
A framework for the selection of plant growth-promoting rhizobacteria based on bacterial competence mechanisms.
Appl. Environ. Microbiol. 2020; 86e00760–00720
Differential priority effects impact taxonomy and functionality of host-associated microbiomes.
Mol. Ecol. 2022; ()
Cultivating the macroalgal holobiont: effects of integrated multi-trophic aquaculture on the microbiome of Ulva rigida (Chlorophyta).
Front. Mar. Sci. 2020; 7: 52
Mariculture of the Asian kelp Undaria pinnatifida and the native kelp Saccharina latissima along the Atlantic coast of Southern Europe: an overview.
Algal Res. 2016; 15: 9-23
Microbiome engineering: synthetic biology of plant-associated microbiomes in sustainable agriculture.
Trends Biotechnol. 2021; 39: 244-261
Increased nutritional value in food crops.
Microb. Biotechnol. 2017; 10: 1004-1007
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://www.cell.com/trends/biotechnology/fulltext/S0167-7799(22)00221-9?rss=yes
- 1
- 2019
- 7
- a
- across
- activating
- Activation
- activity
- affect
- against
- agriculture
- analysis
- and
- Application
- applications
- architecture
- asian
- associated
- authors
- availability
- Bacteria
- based
- beneficial
- benefits
- between
- biology
- Bot
- bull
- capable
- Capacity
- Cause
- causes
- causing
- challenges
- change
- chemical
- China
- Climate
- Climate change
- Coast
- Communities
- community
- comprehensive
- concept
- condition
- Consequences
- consortium
- content
- control
- controlled
- crop
- crops
- cultivation
- Current
- Declining
- Defense
- description
- Detection
- Dev
- Development
- Disease
- diseases
- Diversity
- driven
- Drugs
- dynamic
- dynamics
- Ecosystems
- effects
- efficiency
- efficient
- emerging
- Emerging research
- enemies
- Engineering
- enhancing
- Environment
- environmentally
- Europe
- evolution
- expected
- facilitate
- factors
- farming
- Farms
- food
- forest
- Framework
- from
- Frontiers
- function
- functional
- functionality
- functions
- future
- Gen
- Genetics
- genome
- giant
- Global
- global network
- Globally
- Green
- Growth
- healthy
- help
- host
- HTTPS
- Identification
- Immune system
- immunity
- Impact
- Impacts
- imperative
- importance
- important
- improve
- improved
- in
- india
- indications
- innate
- integrated
- interact
- interaction
- interactions
- isolated
- Isolates
- Key
- knowledge
- known
- large
- leveraging
- lifestyle
- List
- long-term
- Manipulation
- Marine
- Meet
- microbiology
- Microbiome
- mitigation
- model
- native
- Natural
- necessary
- network
- New
- normal
- novel
- ocean
- ONE
- opportunities
- Optimised
- optimized
- organization
- overview
- Pace
- parameters
- performance
- perspective
- Pharmaceutical
- Philippines
- plants
- plato
- Plato Data Intelligence
- PlatoData
- populations
- potential
- predicted
- priority
- Production
- productivity
- Profile
- promotes
- promotion
- prospects
- protection
- Protective
- provide
- quality
- recruitment
- Red
- relationship
- release
- reproduction
- research
- resilience
- Resistance
- resource
- Resources
- review
- Role
- root
- salt
- Scale
- scales
- scenarios
- SCI
- selection
- settlement
- show
- significant
- simplified
- Soft
- Southern
- specific
- State
- statistics
- Status
- Strains
- stress
- structure
- Study
- success
- Surface
- Survey
- Sustainability
- sustainable
- synthetic
- system
- systemic
- targeted
- targets
- taxonomy
- The
- The Future
- The Philippines
- their
- Through
- to
- under
- understanding
- unprecedented
- use
- value
- via
- W
- which
- within
- X
- Yield
- zephyrnet