<!–
–>
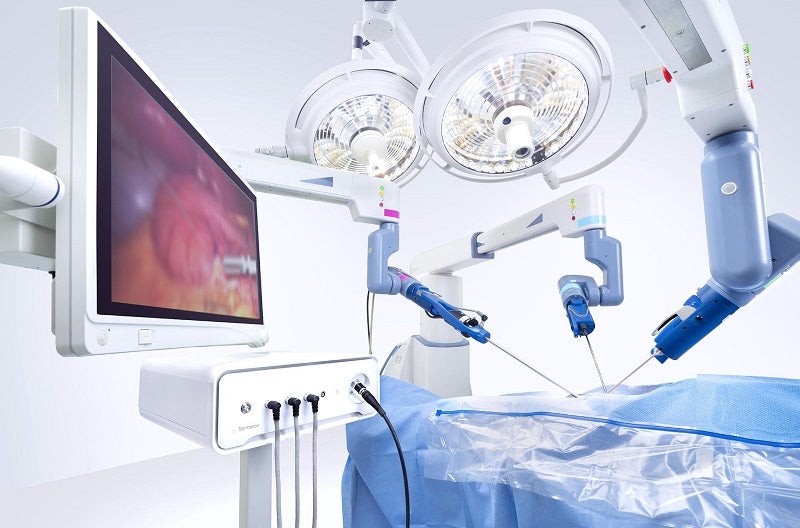
Asensus Surgical has obtained CE mark for the expanded machine vision capabilities on its previously cleared Intelligent Surgical Unit (ISU).
This regulatory approval makes the expanded ISU capabilities available across Japan, the European Union (EU) and the US.
In September 2021, the US Food and Drug Administration (FDA) granted 510(k) clearance for the expanded machine vision capabilities.
The latest approval also covers a review of the Senhance Surgical System platform, which makes it one of the first surgical robotic systems approved through the latest EU Medical Device Regulation (MDR) process.
The expanded capabilities of the ISU include image enhancement, digital tagging, 3D measurement and enhanced camera control based on real-time data from anatomical structures during surgery.
Asensus Surgical president and CEO Anthony Fernando said: “We are thrilled to be able to offer these ground-breaking ISU capabilities to surgeons in the EU.
“Surgeon feedback from the expanded feature set across the US and Japan has been tremendous, and we look forward to partnering with new and existing customers to help bring advanced real-time intraoperative digital tools into operating rooms throughout Europe.
“This is a significant milestone for the Company as our filing included a review of the Senhance Surgical System, both software and hardware, under the new, stricter EU MDR process.”
The company believes that the expanded augmented intelligence features approval will offer better support for surgeons across several specialities and procedures.
It also expects that the foundational elements of the features will allow it to use the ISU’s capabilities and potential to introduce further augmented features in the future.
<!– GPT AdSlot 3 for Ad unit 'Verdict/Verdict_In_Article' ### Size: [[670,220]] —
!– End AdSlot 3 –>
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://www.medicaldevice-network.com/news/asensus-surgical-isu-expanded-capabilities/
- 2021
- 3d
- a
- Able
- across
- Ad
- administration
- advanced
- analytics
- and
- Anthony
- approval
- approved
- augmented
- available
- based
- believes
- Better
- bring
- camera
- capabilities
- ceo
- company
- control
- covers
- credit
- Customers
- data
- device
- digital
- drug
- during
- elements
- enables
- enhanced
- EU
- Europe
- European
- european union
- European Union (EU)
- existing
- expanded
- expects
- fda
- Feature
- Features
- feedback
- Filing
- First
- food
- Food and Drug Administration
- Food and Drug Administration (FDA)
- Forward
- from
- further
- future
- GlobalData
- granted
- ground-breaking
- Hardware
- help
- HTTPS
- image
- in
- include
- included
- Intelligence
- Intelligent
- introduce
- IT
- Japan
- latest
- Look
- machine
- machine vision
- MAKES
- mark
- MDR
- medical
- medical device
- milestone
- New
- obtains
- offer
- ONE
- operating
- partnering
- platform
- plato
- Plato Data Intelligence
- PlatoData
- potential
- powered
- president
- previously
- procedures
- process
- real-time
- real-time data
- Regulation
- regulatory
- regulatory approval
- review
- Robotic
- Rooms
- Said
- September
- set
- several
- Share
- significant
- Size
- Software
- stricter
- support
- Surgery
- surgical
- system
- Systems
- The
- The Future
- thrilled
- Through
- throughout
- to
- tools
- tremendous
- under
- union
- unit
- us
- use
- vision
- which
- will
- zephyrnet