<!–
–>

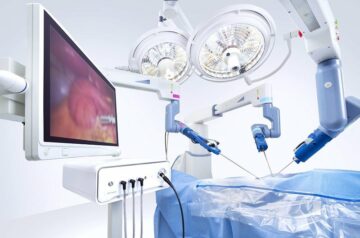
Medtronic has secured CE Mark for its Affera Mapping and Ablation System to treat atrial arrhythmias.
The Affera system uses the Sphere-9 Catheter and the Affera Prism-1 Mapping Software.
It has been designed to both map and ablate atrial arrhythmias, as well as provide real-time feedback through its intuitive mapping and navigation software.
The company stated that with the integration of the Sphere-9 pulsed field ablation (PFA), radiofrequency (RF) and high-density (HD) mapping catheter, the complete system creates a new paradigm in electrophysiology.
Together with the integrated mapping and navigation system, the Sphere-9 Catheter rapidly generates complex electro-anatomical maps.
This allows physicians to provide wide-area focal ablation lesions of choice between RF or PFA, based on the patient and procedure requirements.
The nitinol 9mm ablation tip of the catheter requires fewer focal ablation lesion applications, which reduces the procedure times compared to standard irrigated ablation catheters.
Meanwhile, the mapping software offers an optimised user experience through streamlined insights and feedback.
Medtronic Cardiac Ablation Solutions business vice-president, chief medical officer Khaldoun Tarakji said: “The revolutionary Affera Mapping and Ablation System combined with the novel Sphere-9 Catheter represent a great advancement in the field of HD mapping and focal ablation.
“Current technologies require the use of separate HD mapping and ablation catheters.
“The ability to map, ablate and validate with the Sphere-9 Catheter enables the physician to eliminate the need to exchange catheters and empowers them to choose the energy source, whether RF or PF, based on the patient’s needs.”
The CE Mark is supported by data obtained from clinical trials evaluating the performance and safety of the Sphere-9 Catheter and Mapping System.
Last December, the company completed participant enrolment in the US Food and Drug Administration (FDA) Investigational Device Exemption (IDE) randomised, pivotal Affera SPHERE Per-AF clinical trial.
The trial was designed to assess the Affera Mapping and Ablation System’s effectiveness and safety to treat persistent atrial fibrillation.
Furthermore, Medtronic plans to commercialise the Affera Mapping and Ablation System in Europe from the first half of the year.
<!– GPT AdSlot 3 for Ad unit 'Verdict/Verdict_In_Article' ### Size: [[670,220]] —
!– End AdSlot 3 –>
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://www.medicaldevice-network.com/news/medtronics-affera-mapping-ablation-system/
- :is
- a
- ability
- Ad
- administration
- advancement
- all-in-one
- allows
- and
- applications
- AS
- based
- between
- business
- by
- chief
- choice
- Choose
- Clinical
- clinical trials
- combined
- company
- compared
- complete
- complex
- creates
- credit
- data
- December
- designed
- device
- drug
- effectiveness
- eliminate
- empowers
- enables
- energy
- Europe
- evaluating
- exchange
- experience
- fda
- feedback
- field
- First
- food
- Food and Drug Administration
- Food and Drug Administration (FDA)
- For
- from
- generates
- GlobalData
- great
- Half
- HTTPS
- in
- insights
- integrated
- integration
- intuitive
- ITS
- jpg
- map
- mapping
- Maps
- mark
- medical
- Navigation
- Need
- needs
- New
- novel
- obtained
- of
- Offers
- Officer
- on
- Optimised
- paradigm
- patient
- performance
- physician
- pivotal
- plans
- plato
- Plato Data Intelligence
- PlatoData
- PLC
- PRNewswire
- provide
- rapidly
- real-time
- receives
- reduces
- represent
- require
- Requirements
- requires
- revolutionary
- Safety
- Said
- Secured
- separate
- Share
- Size
- Software
- Solutions
- Source
- standard
- stated
- streamlined
- Supported
- system
- Technologies
- that
- The
- Them
- Through
- times
- tip
- to
- treat
- trial
- trials
- unit
- us
- use
- User
- User Experience
- VALIDATE
- WELL
- whether
- which
- with
- year
- zephyrnet