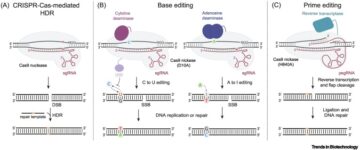
Epithelial cell coculture models for studying infectious diseases: benefits and limitations.
J. Biomed. Biotechnol. 2011; 2011852419
Differential cytokine expression in direct and indirect co-culture of islets and mesenchymal stromal cells.
Cytokine. 2022; 150155779
Microfluidic co-culture platform to recapitulate the maternal–placental–embryonic axis.
Adv. Biol. (Weinh). 2021; 5e2100609
Recent advances on cell-based co-culture strategies for prevascularization in tissue engineering.
Front. Bioeng. Biotechnol. 2021; 9745314
A temperature-based easy-separable (TempEasy) 3D hydrogel coculture system.
Adv. Healthc. Mater. 2022; 11e2102389
Nanothin coculture membranes with tunable pore architecture and thermoresponsive functionality for transfer-printable stem cell-derived cardiac sheets.
ACS Nano. 2015; 9: 10186-10202
A novel 3D indirect co-culture system based on a collagen hydrogel scaffold for enhancing the osteogenesis of stem cells.
J. Mater. Chem. B. 2020; 8: 9481-9491
Coaxial 3D bioprinting of tri-polymer scaffolds to improve the osteogenic and vasculogenic potential of cells in co-culture models.
J. Biomed. Mater. Res. A. 2022; 110: 1077-1089
Biomaterials affect cell–cell interactions in vitro in tissue engineering.
J. Mater. Sci. Technol. 2021; 63: 62-72
The expansion of human ES and iPS cells on porous membranes and proliferating human adipose-derived feeder cells.
Biomaterials. 2010; 31: 8012-8021
Use of porous membranes in tissue barrier and co-culture models.
Lab Chip. 2018; 18: 1671-1689
Transparent, nanoporous, and transferable membrane-based cell–cell paracrine signaling assay.
Adv. Mater. 2015; 27: 1893-1899
Porous nanocrystalline silicon membranes as highly permeable and molecularly thin substrates for cell culture.
Biomaterials. 2010; 31: 5408-5417
An advanced human in vitro co-culture model for translocation studies across the placental barrier.
Sci. Rep. 2018; 8: 5388
Triple co-culture of human alveolar epithelium, endothelium and macrophages for studying the interaction of nanocarriers with the air-blood barrier.
Acta Biomater. 2019; 91: 235-247
Hydrolyzed fumonisin B1 induces less inflammatory responses than fumonisin B1 in the co-culture model of porcine intestinal epithelial and immune cells.
Toxicol. Lett. 2019; 305: 110-116
A novel transwell blood brain barrier model using primary human cells.
Front. Cell. Neurosci. 2019; 13: 230
Ultrasound propagation in the micropores of track membranes.
Appl. Phys. Lett. 2005; 87111911
Co-culture systems-based strategies for articular cartilage tissue engineering.
J. Cell. Physiol. 2018; 233: 1940-1951
Engineered co-culture strategies using stem cells for facilitated chondrogenic differentiation and cartilage repair.
Biotechnol. Bioprocess Eng. 2018; 23: 261-270
Cell culture based in vitro test systems for anticancer drug screening.
Front. Bioeng. Biotechnol. 2020; 8: 322
Addressing patient specificity in the engineering of tumor models.
Front. Bioeng. Biotechnol. 2019; 7: 217
Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing.
Lab Chip. 2018; 18: 486-495
Near-physiological microenvironment simulation on chip to evaluate drug resistance of different loci in tumour mass.
Talanta. 2019; 191: 67-73
Comparison of cytotoxicity evaluation of anticancer drugs between real-time cell analysis and CCK-8 method.
ACS Omega. 2019; 4: 12036-12042
Multilayered cell sheets of cardiac reprogrammed cells for the evaluation of drug cytotoxicity.
Tissue Eng. Regen. Med. 2021; 18: 807-818
A cell lines derived microfluidic liver model for investigation of hepatotoxicity induced by drug–drug interaction.
Biomicrofluidics. 2019; 13024101
Integrating organs-on-chips: multiplexing, scaling, vascularization, and innervation.
Trends Biotechnol. 2020; 38: 99-112
Ultrathin transparent membranes for cellular barrier and co-culture models.
Biofabrication. 2017; 9015019
Human co- and triple-culture model of the alveolar-capillary barrier on a basement membrane mimic.
Tissue Eng. Part C Methods. 2018; 24: 495-503
Finite element modeling to analyze TEER values across silicon nanomembranes.
Biomed. Microdevices. 2018; 20: 11
Ultra-thin, aligned, free-standing nanofiber membranes to recapitulate multi-layered blood vessel/tissue interface for leukocyte infiltration study.
Biomaterials. 2018; 169: 22-34
Ultrathin dual-scale nano- and microporous membranes for vascular transmigration models.
Small. 2019; 15e1804111
A collagen gel-coated, aligned nanofiber membrane for enhanced endothelial barrier function.
Sci. Rep. 2019; 9: 14915
Augmented peripheral nerve regeneration through elastic nerve guidance conduits prepared using a porous PLCL membrane with a 3D printed collagen hydrogel.
Biomater. Sci. 2020; 8: 6261-6271
Novel microfluidic colon with an extracellular matrix membrane.
ACS Biomater. Sci. Eng. 2018; 4: 1377-1385
Fabrication of artificial nanobasement membranes for cell compartmentalization in 3D tissues.
Small. 2020; 16: 1907434
Microfluidic gel patterning method by use of a temporary membrane for organ-on-chip applications.
Adv. Mater. Technol. 2018; 3: 1700200
The use of bacterial cellulose as a basement membrane improves the plausibility of the static in vitro blood–brain barrier model.
Int. J. Biol. Macromol. 2019; 126: 1002-1013
Ultrathin silicon membranes for in situ optical analysis of nanoparticle translocation across a human blood–brain barrier model.
ACS Nano. 2020; 14: 1111-1122
Transwell-integrated 2 μm thick transparent polydimethylsiloxane membranes with controlled pore sizes and distribution to model the blood–brain barrier.
Adv. Mater. Technol. 2021; 6: 2100138
3D in vitro human organ mimicry devices for drug discovery, development, and assessment.
Adv. Polym. Technol. 2020; 2020: 6187048
Second-generation lung-on-a-chip with an array of stretchable alveoli made with a biological membrane.
Commun. Biol. 2021; 4: 168
Use of elastic, porous, and ultrathin co-culture membranes to control the endothelial barrier function via cell alignment.
Adv. Funct. Mater. 2021; 31: 2008172
Use of nanosphere self-assembly to pattern nanoporous membranes for the study of extracellular vesicles.
Nanoscale Adv. 2020; 2: 4427-4436
Native extracellular matrix-derived semipermeable, optically transparent, and inexpensive membrane inserts for microfluidic cell culture.
Lab Chip. 2017; 17: 3146-3158
Cellular layer-by-layer coculture platform using biodegradable, nanoarchitectured membranes for stem cell therapy.
Chem. Mater. 2017; 29: 5134-5147
Cardiac-mimetic cell-culture system for direct cardiac reprogramming.
Theranostics. 2019; 9: 6734-6744
Prevascularized, multiple-layered cell sheets of direct cardiac reprogrammed cells for cardiac repair.
Biomater. Sci. 2020; 8: 4508-4520
Novel platform of cardiomyocyte culture and coculture via fibroblast-derived matrix-coupled aligned electrospun nanofiber.
ACS Appl. Mater. Interfaces. 2017; 9: 224-235
Layer-by-layer bioassembly of cellularized polylactic acid porous membranes for bone tissue engineering.
J. Mater. Sci. Mater. Med. 2017; 28: 78
Fabrication of co-cultured tissue constructs using a dual cell seeding compatible cell culture insert with a clip-on scaffold for potential regenerative medicine and toxicological screening applications.
J. Sci. Adv. Mater. Devices. 2020; 5: 207-217
Novel method for the fabrication of ultrathin, free-standing and porous polymer membranes for retinal tissue engineering.
J. Mater. Chem. B. 2017; 5: 5616-5622
Advances in tissue engineering through stem cell-based co-culture.
J. Tissue Eng. Regen. Med. 2015; 9: 488-503
Advances in micro/nanoporous membranes for biomedical engineering.
Adv. Healthc. Mater. 2021; 10e2001545
Robust and gradient thickness porous membranes for in vitro modeling of physiological barriers.
Adv. Mater. Technol. 2020; 52000474
Co-culture of human induced pluripotent stem cell-derived retinal pigment epithelial cells and endothelial cells on double collagen-coated honeycomb films.
Acta Biomater. 2020; 101: 327-343
Nanoporous silicon nitride membranes fabricated from porous nanocrystalline silicon templates.
Nanoscale. 2014; 6: 10798-10805
Second generation nanoporous silicon nitride membranes for high toxin clearance and small format hemodialysis.
Adv. Healthc. Mater. 2020; 9e1900750
Endothelial vacuolization induced by highly permeable silicon membranes.
Acta Biomater. 2014; 10: 4670-4677
A silicon nanomembrane platform for the visualization of immune cell trafficking across the human blood–brain barrier under flow.
J. Cereb. Blood Flow Metab. 2019; 39: 395-410
Porous substrates promote endothelial migration at the expense of fibronectin fibrillogenesis.
ACS Biomater. Sci. Eng. 2018; 4: 222-230
Micropatterned poly(ethylene glycol) islands disrupt endothelial cell–substrate interactions differently from microporous membranes.
ACS Biomater. Sci. Eng. 2020; 6: 959-968
A review on porous polymeric membrane preparation. Part I. Production techniques with polysulfone and poly(vinylidene fluoride).
Polymers (Basel). 2019; 11: 1160
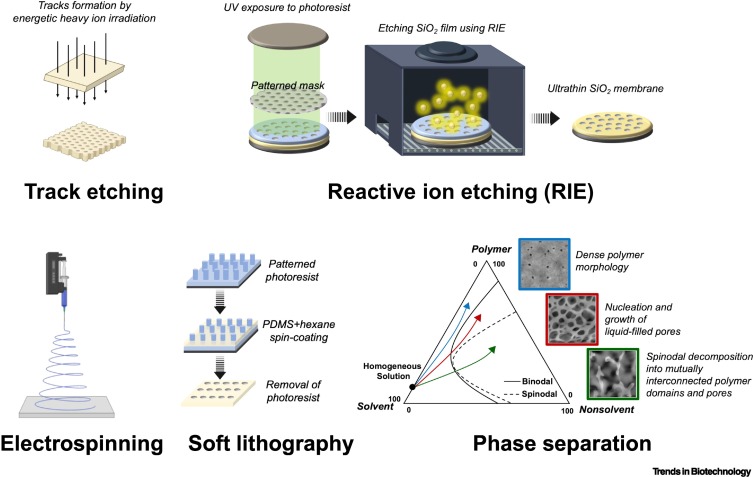
Track etching technique in membrane technology.
Radiat. Meas. 2001; 34: 559-566
Microfluidic isolation of highly pure embryonic stem cells using feeder-separated co-culture system.
Sci. Rep. 2013; 3: 2433
Microfabricated tuneable and transferable porous PDMS membranes for organs-on-chips.
Sci. Rep. 2018; 8: 13524
Organ-on-a-chip: recent breakthroughs and future prospects.
Biomed. Eng. Online. 2020; 19: 9
Porous membranes in secondary battery technologies.
Chem. Soc. Rev. 2017; 46: 2199-2236
Large-scale fabrication of free-standing and sub-μm PDMS through-hole membranes.
Nanoscale. 2018; 10: 7711-7718
Composition and phase changes observed by magnetic resonance imaging during non-solvent induced coagulation of cellulose.
Polymer. 2002; 43: 5827-5837
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://www.cell.com/trends/biotechnology/fulltext/S0167-7799(22)00196-2?rss=yes
- 1
- 3d
- 7
- a
- across
- advanced
- advances
- affect
- aligned
- analysis
- analyze
- and
- applications
- architecture
- Array
- artificial
- assessment
- authors
- Axis
- barrier
- barriers
- based
- battery
- benefits
- between
- biomedical
- blood
- BONE
- Brain
- breakthroughs
- Cells
- Changes
- chip
- compatible
- control
- controlled
- Culture
- Derived
- Development
- Devices
- different
- direct
- discovery
- diseases
- Disrupt
- distribution
- double
- drug
- drug discovery
- drug testing
- Drugs
- during
- Engineering
- enhanced
- enhancing
- evaluate
- evaluation
- expansion
- films
- flow
- format
- from
- function
- functionality
- future
- generation
- High
- highly
- HTTPS
- human
- Imaging
- improve
- improves
- in
- Infectious diseases
- Inserts
- interaction
- interactions
- Interface
- interfaces
- investigation
- Islands
- isolation
- limitations
- lines
- List
- Liver
- made
- Mass
- Matrix
- medicine
- method
- methods
- migration
- model
- modeling
- models
- multi-layered
- nano
- novel
- online
- part
- patient
- Pattern
- phase
- platform
- plato
- Plato Data Intelligence
- PlatoData
- polymer
- potential
- prepared
- primary
- Production
- promote
- prospects
- real-time
- recent
- regenerative
- repair
- Resistance
- resonance
- review
- scaling
- SCI
- screening
- secondary
- Silicon
- simulation
- sizes
- small
- specificity
- Stem
- stem cells
- strategies
- studies
- Study
- Studying
- Supported
- system
- Systems
- techniques
- Technologies
- Technology
- templates
- temporary
- test
- Testing
- The
- therapy
- Through
- tissues
- to
- track
- trafficking
- transparent
- under
- use
- Values
- via
- visualization
- vivo
- W
- X
- zephyrnet