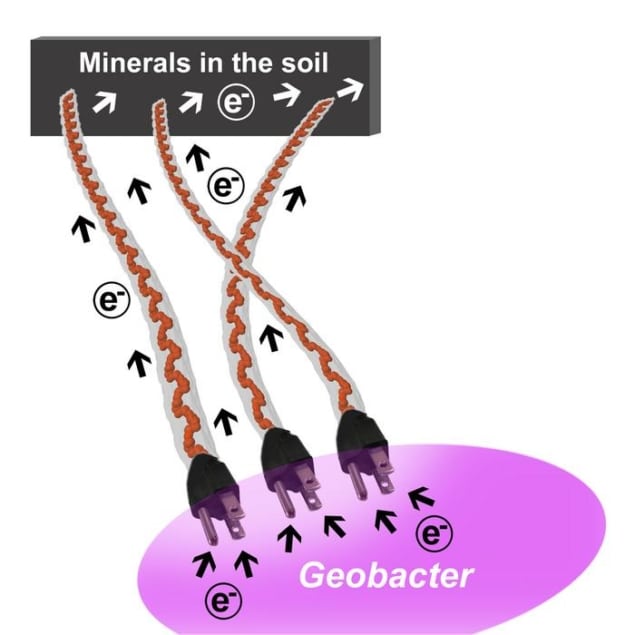
Because there’s not much oxygen deep underground, the bacteria that live there have evolved other ways to get rid of the electrons they produce when they “breathe”. One of these workarounds involves sending out conductive filaments – nanowires – into the soil to disperse the electrons, but important details of this process have eluded biophysicists’ understanding.
Researchers at Yale University, US and NOVA University Lisbon in Portugal have now found that for bacteria in the genus Geobacter, a single protein family acts like a series of electrically connecting “plugs” for charging these microbial nanowires. The finding greatly simplifies the model of how these bacteria export electrons, and the team say this “minimal wiring machinery” may be common among bacterial species.
Bacteria that live in soil have two ways of donating the electrons they produce to external electron acceptors. The first involves transferring the electrons to soil minerals and is known as extracellular electron transfer (EET). The second, direct interspecies electron transfer (DIET), involves partner species. Both processes are vital for the microbes’ ability to survive and form communities, but they can be inefficient. Bacteria like Geobacter have therefore evolved to produce conductive nanowires that facilitate faster, long-range EET.
Five proteins
The protein family the Yale–NOVA team identified as key to the operation of these nanowires contains five proteins. All of them reside in the space between the bacteria’s inner and outer membrane – the bacterial periplasm – and they are known as periplasmic cytochrome ABCDE (PpcA-E). These proteins inject electrons into filaments on bacterial surfaces that act as nanowires, creating an electric connection for “metal breathing” Geobacter.
This electrical connection allows Geobacter to transfer excess electrons produced during metabolism to minerals in soil without the need for intermediaries, explains Yale’s Nikhil Malvankar, who co-led the study with Carlos Salgueiro at NOVA. In essence, the proteins act as plugs within a natural soil-based “electrical grid”. This grid may be responsible for allowing many types of microbes to survive and support life, the researchers say.
Microscopic pistons push filaments made of cytochromes
Though bacterial filaments were first observed in 2002, scientists initially thought they were made up of so-called pili proteins (“pili” means “hairs” in Latin). Many bacteria do have pili on their surface, and genetic data suggested these hairlike filaments could play a similar role in Geobacter, Malvankar says. In 2021, however, researchers in Malvankar’s lab solved the atomic structure of pili and showed that they instead act as pistons that push filaments made up of cytochromes. In addition, the atomic structures of cytochromes known as OmcS and OmcZ includes a chain of metal-containing heme molecules that carry electrons (red in the image above).
While these atomic structures explained how nanowires transport electrons, the connection between the nanowires and the bacteria’s surface remained a mystery, he adds. This is because most cell surfaces are electrically non-conducting.
“It was thought that another family of proteins embedded in the bacterial membrane, called porin cytochromes, was responsible for this connection despite bacteria being able to transmit electricity even in their absence,” Malvankar explains. “The presence of periplasmic proteins transferring electrons to nanowires eliminates the need for any intermediate electron carriers and explains how cells transmit electrons at a remarkably fast rate (a million electrons per second), even though electrons in proteins can move at rates at least 10 times slower.”
Working out the relationship between PpcA-E and OmcS
The researchers began by measuring the energy of electrons in OmcS. They found it was the same as in PpcA-E, which team member Catharine Shipps says was surprising because the OmcS measurement was expected to differ by 0.1 V. “At the time of the first measurements on OmcS (in 2011), we did not know that OmcS formed nanowires,” says Shipps, who performed this part of the work. “These previous measurements were made by treating the cytochromes as non-filamentous, something that could explain this large discrepancy.”
In 2015, Salgueiro and colleagues at NOVA hypothesised that PpcA-Es could transfer electrons to OmcS. However, testing this hypothesis was not feasible at the time because of the difficulty in obtaining purified OmcS nanowires. Malvankar says that Shipps’ finding added to the picture by suggesting that PpcA-E could donate electrons directly to OmcS – something that another team member, Vishok Srikanth, proposed after noticing that OmcS and PpcA-E stay together when extracted from bacteria. “All these results led us to propose that PpcA-E could pass electrons to nanowires,” he says. The two groups then confirmed their hypothesis using nuclear magnetic resonance spectroscopy.

Microbial nanowires create ‘electronic nose’ for health monitoring
“Our discovery greatly simplifies the model of how bacteria export electrons by overcoming slow electron flow among individual proteins,” Malvankar tells Physics World. “The discovery by another of our team members, Cong Shen, that this protein family is evolutionary and conserved across many species, not just Geobacter, means that this minimal wiring machinery could be ubiquitous in many bacteria.”
The researchers, who report their work in Nature Communications, are now engineering the newly discovered mechanism into bacteria that are important for the climate or capable of making biofuels. The aim is to help these beneficial organisms grow faster. “We are also working on how another nanowire of cytochrome OmcZ is charged and identifying the role of porin-cytochromes in these processes,” Malvankar says.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://physicsworld.com/a/bacterial-nanowires-make-an-electrical-grid-in-the-soil/
- :is
- :not
- $UP
- 1
- 10
- 160
- 2011
- 2015
- 2021
- 90
- a
- ability
- Able
- above
- absence
- across
- Act
- acts
- added
- addition
- Adds
- After
- aim
- All
- Allowing
- allows
- also
- among
- an
- and
- Another
- any
- ARE
- AS
- At
- Bacteria
- BE
- because
- began
- being
- beneficial
- between
- both
- but
- by
- cables
- called
- CAN
- capable
- carriers
- carry
- cell
- Cells
- chain
- charged
- charging
- Circle
- Climate
- colleagues
- Common
- Communities
- CONFIRMED
- connected
- Connecting
- connection
- contains
- could
- courtesy
- create
- Creating
- data
- deep
- Despite
- details
- diagram
- DID
- Diet
- differ
- Difficulty
- direct
- directly
- discovered
- discovery
- discrepancy
- do
- donate
- donating
- during
- Electric
- electricity
- electrons
- eliminates
- embedded
- energy
- Engineering
- eric
- essence
- Even
- evolved
- excess
- expected
- Explain
- explained
- Explains
- export
- external
- facilitate
- family
- FAST
- faster
- feasible
- finding
- First
- five
- flow
- For
- form
- formed
- found
- from
- genetic
- get
- greatly
- Grid
- Group’s
- Grow
- Have
- he
- Health
- help
- How
- However
- HTTPS
- identified
- identifying
- image
- important
- in
- includes
- individual
- inefficient
- information
- initially
- inject
- inner
- instead
- intermediaries
- Intermediate
- into
- involves
- issue
- IT
- jpg
- just
- Key
- Know
- known
- lab
- large
- Latin
- least
- Led
- Life
- like
- live
- machinery
- made
- make
- Making
- many
- max-width
- May..
- means
- measurement
- measurements
- measuring
- mechanism
- member
- Members
- million
- minerals
- minimal
- model
- most
- move
- much
- Mystery
- Natural
- Nature
- Need
- newly
- now
- nuclear
- observed
- obtaining
- of
- on
- ONE
- operation
- or
- organisms
- Other
- our
- out
- overcoming
- Oxygen
- part
- partner
- pass
- per
- performed
- Physics
- Physics World
- picture
- pink
- plato
- Plato Data Intelligence
- PlatoData
- Play
- Portugal
- power
- presence
- previous
- process
- processes
- produce
- Produced
- propose
- proposed
- Protein
- Proteins
- Push
- Rate
- Rates
- Red
- relationship
- remained
- report
- represented
- researchers
- resonance
- responsible
- Results
- Rid
- Role
- same
- say
- says
- scientists
- Second
- sending
- Series
- showed
- showing
- similar
- simplifies
- single
- slow
- slower
- soil
- something
- Space
- Spectroscopy
- stay
- structure
- structures
- Study
- support
- Surface
- surprising
- survive
- team
- Team members
- tells
- Testing
- that
- The
- their
- Them
- then
- There.
- therefore
- These
- they
- this
- though?
- thought
- thumbnail
- time
- times
- to
- together
- transfer
- Transferring
- transmit
- transport
- treating
- true
- two
- types
- ubiquitous
- underground
- understanding
- university
- us
- using
- via
- vital
- was
- ways
- we
- were
- when
- which
- WHO
- with
- within
- without
- Work
- working
- world
- zephyrnet