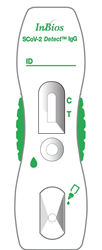
InBios SCoV-2 Detect IgG Rapid Test
SEATTLE (PRWEB) September 15, 2021
InBios International Inc., a leading developer of diagnostic tests for emerging infectious diseases, announced today that it received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for its SCoV-2 Detect IgG Rapid Test, a qualitative immunoassay for the detection of IgG antibodies to SARS-CoV-2. This marks InBios’ fifth EUA granted for SARS-CoV-2 diagnostics since the beginning of the COVID-19 pandemic.
The test can be performed using serum, plasma (sodium citrate, dipotassium EDTA, or sodium heparin treated), venous whole blood (sodium citrate, dipotassium EDTA or sodium heparin treated) or fingerstick whole blood samples and takes about 20 minutes to obtain results.
“As we see a resurgence of COVID-19, it’s vital to arm healthcare workers with an accurate, reliable test for rapid SARS-CoV-2 antibody detection,” said Dr. Syamal Raychaudhuri, InBios’ chief scientific officer. “Our IgG antibody test can also help public health officials understand more about how the virus spreads within a community. We’re pleased that our suite of COVID-19 diagnostics is providing critical insight into this devastating disease and that we have increased capacity to manufacture tests to meet worldwide demand.”
In a clinical evaluation at point-of-care settings using fingerstick whole blood of individuals who were 15 days or more post symptom onset, InBios’ SCoV-2 Detect IgG Rapid Test accurately determined 96.9% (PPA) of those who were positive and 100% of those who were negative (NPA) for SARS-CoV-2 IgG antibodies. The test, which is available to order immediately, includes all reagents and controls required to run 50 test specimens with results in approximately 20 minutes.
The SCoV-2 Detect IgG Rapid Test is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. The SCoV-2 Detect IgG Rapid Test should not be used to diagnose or exclude acute SARS-CoV-2 infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity.
Testing of serum, plasma, and venous whole blood specimens is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C 263a, that meet the requirements to perform high or moderate complexity tests. Testing of fingerstick whole blood specimens is limited to laboratories certified under CLIA that meet the requirements to perform high, moderate or waived complexity tests. Fingerstick whole blood specimens testing is also authorized for use in point-of-care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
While the SCoV-2 Detect IgG Rapid Test has not been FDA cleared or approved, it has been authorized for emergency use by FDA under an EUA for use by authorized laboratories. This product has been authorized only for detecting the presence of IgG antibodies to SARS-CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
Funding to achieve EUA for the SCoV-2 Detect IgG Rapid Test comes in part from Department of Health and Human Services (HHS) Biomedical Advanced Research and Development Authority (BARDA) within the Office of the Assistant Secretary for Preparedness and Response, and the U.S. Army Medical Material Development Activity (USAMMDA) Warfighter Protection and Acute Care (WPAC) Program Management Office (with funding provided by the Defense Health Agency (DHA) through the CARES Act), under contracts 75A50120C00090 and W81XWH-16-D-0009, respectively.
For more information about InBios COVID-19 tests, visit: http://www.inbios.com/covid-19/.
For more information on COVID-19, please visit http://www.cdc.gov or http://www.who.int.
About InBios: InBios International Inc. specializes in the design, development and manufacture of diagnostic assays for emerging infectious diseases and biothreats. Located in Seattle, Washington, InBios offers superior quality products which are accurate, easy to use and cost effective. InBios is GMP compliant, FDA registered, USDA licensed and ISO 13485:2016 certified. For more information, visit http://www.inbios.com.
Share article on social media or email:
- 2016
- 7
- All
- announced
- ARM
- article
- Assistant
- authorization
- blood
- Capacity
- care
- CARES Act
- certificate
- chief
- community
- compliance
- contracts
- COVID-19
- COVID-19 pandemic
- Defense
- Demand
- Design
- Detection
- Developer
- Development
- Disease
- diseases
- drug
- Effective
- fda
- Federal
- food
- funding
- Health
- healthcare
- High
- How
- HTTPS
- image
- Inc.
- Infectious diseases
- information
- International
- IT
- leading
- Limited
- Long
- management
- Media
- medical
- Offers
- Officer
- operating
- order
- Other
- pandemic
- Product
- Products
- Program
- protection
- Protective
- public
- public health
- quality
- Requirements
- research
- research and development
- response
- Results
- Run
- Services
- Social
- social media
- specializes
- test
- Testing
- tests
- time
- u.s.
- usda
- virus
- viruses
- washington
- WHO
- within
- workers
- worldwide