- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
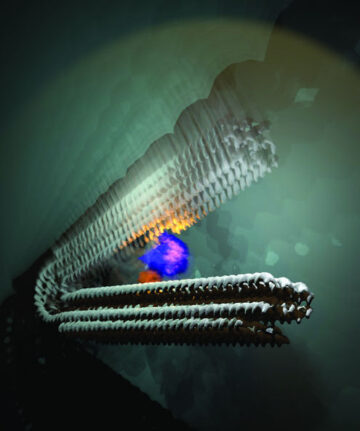
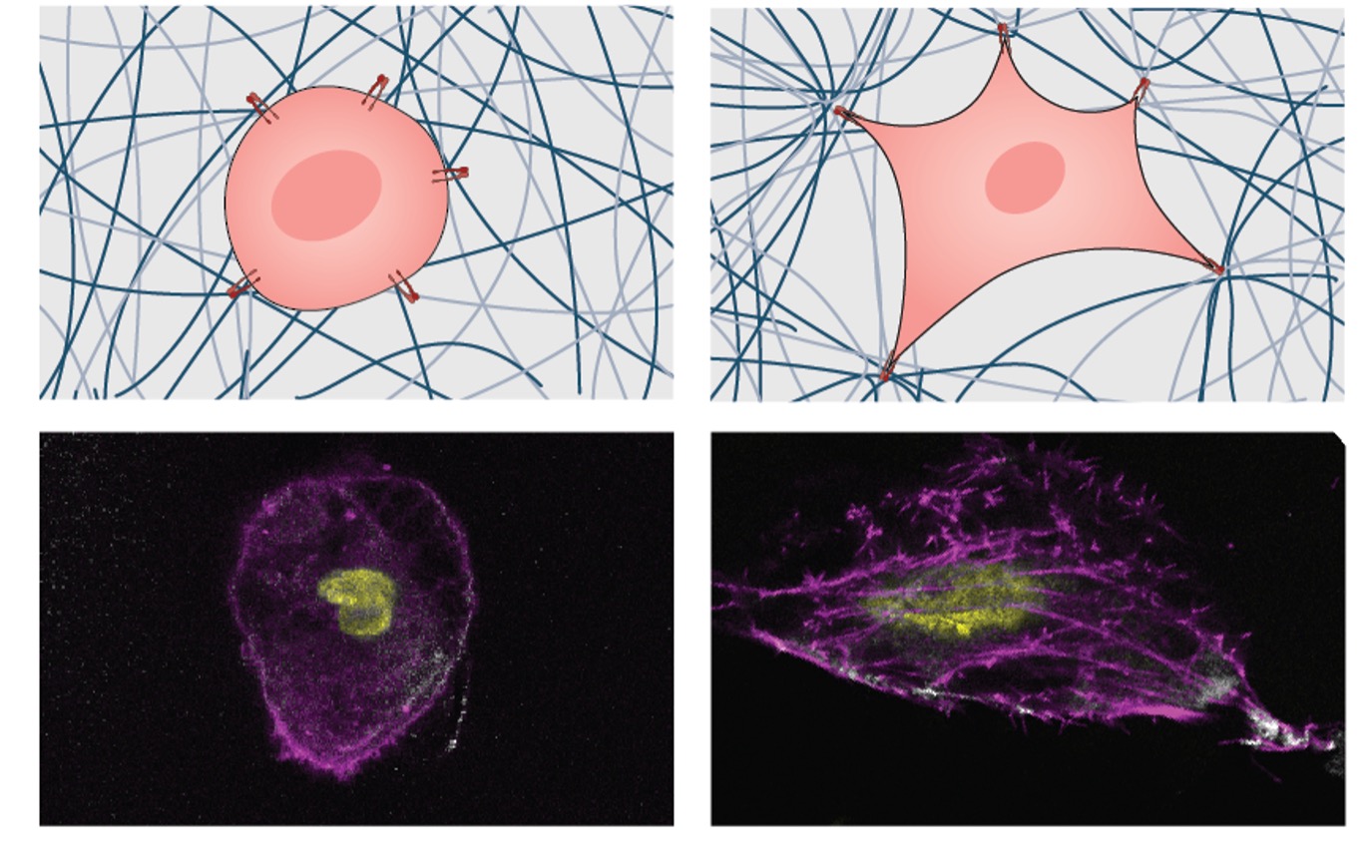
- Source: https://www.nanowerk.com/nanotechnology-news3/newsid=64680.php
- :is
- :where
- $UP
- 10
- 19
- 2%
- 2024
- 3d
- 4
- 5
- 6
- 7
- 8
- 9
- a
- Able
- About
- accelerated
- Activation
- adding
- affects
- aggressive
- All
- also
- alterations
- amazing
- an
- and
- any
- anything
- ARE
- AS
- Assistant
- assisted
- At
- BE
- because
- been
- behave
- behavior
- Better
- biology
- Biomaterials
- biomedical
- body
- builders
- Building
- but
- by
- called
- CAN
- Cancer
- Cancer cells
- capable
- cascade
- cell
- Cells
- cellular
- Center
- change
- Changes
- changing
- class
- collaborators
- come
- Communication
- complex
- construction
- context
- control
- could
- courtesy
- crew
- damage
- Date
- Department
- developed
- different
- Director
- discovery
- Disease
- distinct
- do
- down
- dr
- driven
- else
- encourage
- Engineering
- enhanced
- Environment
- Ether (ETH)
- Even
- exciting
- exemplifies
- exhibited
- explained
- explore
- Exploring
- fascinating
- feel
- fight
- Fix
- For
- fostered
- found
- functions
- future
- Genetics
- get
- gets
- Give
- Growth
- happened
- harder
- Have
- help
- here
- hope
- How
- How To
- HTTPS
- if
- image
- imagine
- important
- in
- include
- increased
- incredibly
- influence
- initiate
- inside
- Institute
- instructions
- interact
- interesting
- into
- intricate
- introduced
- IT
- journal
- jpg
- language
- leading
- learning
- like
- make
- Making
- manipulating
- materials
- Matrix
- means
- mechanical
- mechanism
- Middle
- might
- more
- move
- nascent
- New
- now
- observed
- of
- Offers
- on
- open
- or
- Other
- phenomenon
- PHP
- plato
- Plato Data Intelligence
- PlatoData
- power
- Prevention
- process
- Production
- Professor
- Programs
- promotes
- properties
- published
- RE
- ready
- recent
- Regulation
- regulatory
- repair
- repairing
- repairs
- research
- researchers
- Respond
- responses
- s
- Scholar
- scientists
- send
- sense
- Shape
- Signal
- signals
- Simple
- simply
- site
- slow
- So
- something
- speak
- special
- specialized
- stated
- Stem
- stem cells
- Still
- structures
- Study
- support
- Surrounding
- team
- terms
- texas
- that
- The
- their
- Them
- There.
- These
- they
- this
- tiny
- tissue
- to
- tools
- transformed
- treat
- trigger
- triggering
- Turning
- type
- types
- typically
- understand
- underwent
- university
- using
- utilized
- was
- ways
- we
- WELL
- were
- What
- when
- whereas
- which
- while
- with
- without
- workers
- world
- Your
- zephyrnet
More from Nanowerk
Ultrasmall swirling magnetic vortices detected in iron-containing material
Source Node: 2043521
Time Stamp: Apr 2, 2023
New techniques efficiently accelerate sparse tensors for massive AI models
Source Node: 2359297
Time Stamp: Oct 31, 2023
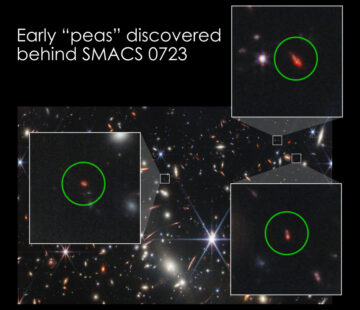
James Webb Space Telescope reveals links between galaxies near and far
Source Node: 1887001
Time Stamp: Jan 9, 2023
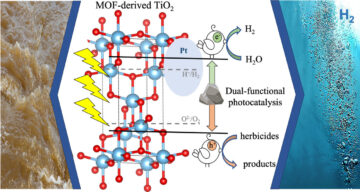
MOF catalyst purifies herbicide-tainted water and produces hydrogen
Source Node: 1995631
Time Stamp: Mar 6, 2023
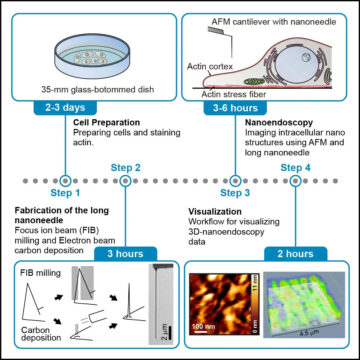
Researchers define a protocol for narrow cantilever fabrication and high-resolution imaging of living cells using AFM
Source Node: 2253898
Time Stamp: Sep 5, 2023
AI protects power grid from fluctuations caused by renewables and EVs
Source Node: 2498300
Time Stamp: Feb 28, 2024
ChatGPT writes convincing fake scientific abstracts that fool reviewers in study
Source Node: 1899963
Time Stamp: Jan 16, 2023
Biosensor could lead to new drugs, sensory organs on a chip
Source Node: 1945808
Time Stamp: Feb 8, 2023
Nanopore-based sensing device explores neurodegenerative diseases
Source Node: 1889038
Time Stamp: Jan 10, 2023
An ink for 3D-printing flexible devices without mechanical joints
Source Node: 2550106
Time Stamp: Apr 18, 2024
Researchers develop selective transfer printing technology for microLEDs
Source Node: 2415981
Time Stamp: Dec 26, 2023