One-atom thick materials like graphene (a 2D sheet of carbon) conduct protons extremely well but they become impermeable to protons the thicker they get. Indeed, 2D molybdenum sulphide (MoS2) becomes completely impermeable to protons at just three atoms thick. A team of researchers from the UK, China and Belgium has now found that materials known as ion-exchanged micas are highly efficient proton conductors even when they are ten atoms thick. This surprising new result could prove to be important for applications such as fuel cells and other hydrogen-related technologies.
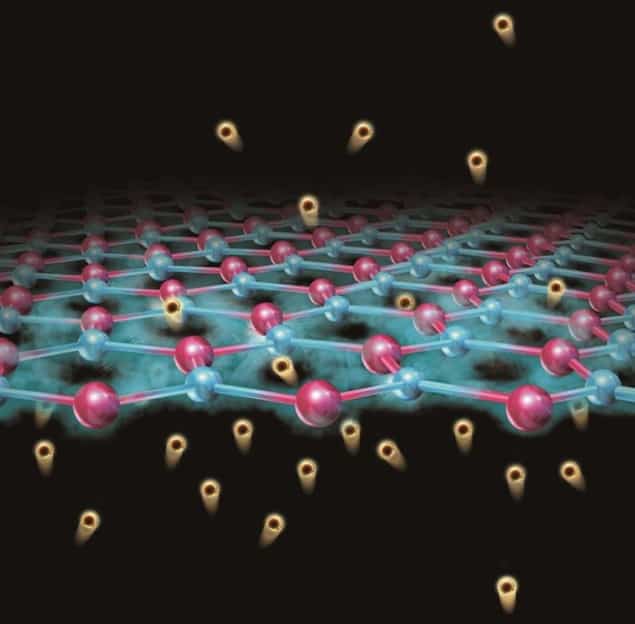
Micas (a type of mineral commonly found in soil) are made up of aluminosilicate layers that are normally covered with cations, such as potassium ions (K+). These native ions can readily be exchanged for other ions, like protons (H+), lithium (Li+) or caesium (Cs+). It is particularly easy to substitute H+ for the native ions.
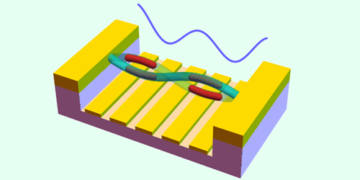
Aluminosilicate slabs pierced by tubular channels
As well as being efficient proton exchangers, micas also have a relatively sparse crystal structure that contains hexagonal rings roughly 5.2 angstroms in size in the basal plane. These rings are much bigger than those that make up graphene or MoS2, for example, which are around 2.5 and 3.2 angstroms respectively. “We can thus think of micas as aluminosilicate slabs pierced by tubular channels,” says Marcelo Lozada-Hidalgo of the University of Manchester, who co-led this research effort together with Nobel laureate Andre Geim. “These channels aren’t empty but filled with hydroxyl (OH–) groups that resemble the proton-conducting 1D chains in water, so they are thus permeable to protons.”
The researchers studied two types of micas (known as muscovite and vermiculite), which are made up of silicon-oxygen (Si-O) and aluminium-oxygen (Al-O) octahedra that form a 2D sheet. They were able to prepare atomically thin samples of these materials by shaving off single layers using the now-famous mechanical exfoliation technique – the same that was used to first prepare graphene back in 2004. They then immersed these sheets in 0.1 M caesium nitrate solution at 80°C for a week so that Cs+ ions could exchange for the native ions through the entire volume of the material.
Proton conductivity up to 100 times higher than in graphene
Lozada-Hidalgo and colleagues chose to exchange the native ions for Cs+ in their study because Cs+ is a heavy ion that provides high contrast in subsequent scanning tunnelling microscopy imaging experiments. This means that they could more easily observe how protons exchange for the Cs+ ions when the materials are placed in a humid hydrogen atmosphere later on.
Thanks to electrical- and direct proton flux- measurements (using mass spectrometry), the researchers found that proton conductivity in atomically thin micas is 10 to 100 times higher than in graphene. “This result was surprising since even the thinnest micas are almost 10 times thicker than graphene,” explains project lead author Lucas Mogg of the University of Manchester. “This is encouraging because graphene is already being considered as a promising proton conducting material. Our results show micas could be even more promising – not least because they are abundant and inexpensive.”
Like sponges
“Micas behave essentially like sponges,” he continues. “In our experiments we made these sponges release the Cs+ ions they initially had and instead made them adsorb protons.”
The mica membranes are extremely efficient proton conductors because the big Cs+ ions initially present that “block” the pores in the sponges are replaced with protons – which are the smallest possible ions, he says. These protons take up much less space and essentially unblock the pores, allowing for proton flow.
“Our result also implies that many other 2D materials that aren’t ionic conductors could be made so by using this strategy,” adds Geim. “Many more 2D crystals with similar nanoscale channels could be explored, hopefully bringing unexpected phenomena and new applications in the field of proton and ionic conductors.”
Frictionless flow in 2D channels
Proton conductivity in traditionally inaccessible temperature range
And that is not all: the researchers found that the micas remain efficient conductors of protons between 100°C and 500°C – the temperature range at which fuel cells and other hydrogen-related technologies need to operate and which has been traditionally inaccessible with other such proton-conducting materials until now. “We found that the areal proton conductivity of our mica membranes can be greater than 100 S/cm2 at 500°C, which is well above the current requirements for industry,” says Lozada-Hidalgo.
“Although our devices are only at the proof-of-concept stage at the moment and significant research and development is still required before we can make industrial prototypes, micas are certainly worth investigating for such applications,” he says.
The team, which includes researchers from the Dalian University of Technology and Tianjin University, both in China, and the University of Antwerp in Belgium, is now busy working on building a mica prototype membrane that is big enough to be tested in industrial conditions.
The researchers are also looking into how the proton conductivity, and indeed other mass transport phenomena, changes when the micas are functionalized and their composition varied.
Full details of the present work are reported in Nature Nanotechnology 10.1038/s41565-019-0536-5.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Automotive / EVs, Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- ChartPrime. Elevate your Trading Game with ChartPrime. Access Here.
- BlockOffsets. Modernizing Environmental Offset Ownership. Access Here.
- Source: https://physicsworld.com/a/thick-mica-membranes-make-excellent-proton-conductors/
- :has
- :is
- :not
- $UP
- 1
- 10
- 100
- 2D
- 2D materials
- 90
- a
- Able
- above
- abundant
- AC
- Adds
- All
- Allowing
- already
- also
- and
- applications
- ARE
- around
- AS
- At
- Atmosphere
- author
- back
- BE
- because
- become
- becomes
- been
- before
- behave
- being
- Belgium
- between
- Big
- bigger
- both
- Bringing
- Building
- busy
- but
- by
- CAN
- carbon
- Cells
- certainly
- chains
- Changes
- channels
- China
- chose
- colleagues
- commonly
- completely
- composition
- conditions
- Conduct
- conducting
- conductivity
- considered
- contains
- continues
- contrast
- could
- covered
- Crystal
- Current
- details
- Development
- Devices
- direct
- easily
- easy
- efficient
- effort
- encouraging
- enough
- Entire
- essentially
- Even
- example
- excellent
- exchange
- exchanged
- exchangers
- experiments
- Explains
- Explored
- extremely
- field
- filled
- First
- flow
- For
- form
- found
- from
- Fuel
- fuel cells
- GAS
- get
- Graphene
- greater
- Group’s
- had
- Have
- he
- heavy
- High
- higher
- highly
- Hopefully
- How
- HTML
- http
- HTTPS
- humid
- hydrogen
- image
- Imaging
- immersed
- important
- in
- inaccessible
- includes
- indeed
- industrial
- industry
- inexpensive
- information
- initially
- instead
- into
- investigating
- Ionic
- issue
- IT
- jpg
- just
- known
- later
- layers
- lead
- least
- less
- like
- lithium
- looking
- made
- make
- manchester
- many
- Mass
- material
- materials
- max-width
- means
- measurements
- mechanical
- MiCA
- Microscopy
- mineral
- moment
- more
- much
- native
- Nature
- Need
- New
- nobel
- Nobel Laureate
- normally
- now
- observe
- of
- off
- on
- only
- operate
- or
- Other
- our
- particle
- particularly
- Physics
- Physics World
- plato
- Plato Data Intelligence
- PlatoData
- possible
- Prepare
- present
- project
- promising
- protons
- prototype
- prototypes
- Prove
- provides
- range
- relatively
- release
- remain
- replaced
- Reported
- required
- Requirements
- research
- research and development
- researchers
- result
- Results
- roughly
- same
- says
- scanning
- sheet
- show
- showing
- significant
- similar
- since
- single
- Size
- smallest
- So
- soil
- solution
- Space
- Stage
- Still
- Strategy
- structure
- studied
- Study
- subsequent
- such
- surprising
- Take
- team
- Technologies
- Technology
- ten
- tested
- than
- that
- The
- the UK
- their
- Them
- then
- These
- they
- Think
- this
- those
- three
- Through
- thumbnail
- Thus
- times
- to
- together
- traditionally
- transport
- true
- two
- type
- types
- Uk
- Unexpected
- university
- until
- used
- using
- volume
- was
- Water
- we
- week
- WELL
- were
- when
- which
- WHO
- with
- Work
- working
- world
- worth
- zephyrnet