IEC 82304, along with the IEC 62304, is an essential standard for software-based medical devices. Although the two IEC standards may look very similar, they are actually different in terms of requirements and application.
We have been discussing quite extensively on the IEC 62304 and the related documentation needed for an accurate software development lifecycle: software development plan, software architecture, software test protocols and reports are only few of the documents specifically required by IEC 62304.
In this article, we will deal with the requirements mentioned on IEC 82304, highlighting the link with the IEC 62304 and discussing in details the documents needed to reach compliance with this standard.
Currently, the IEC 82304 is not a harmonised standard to the EU MDR and IVDR, thus its application is not considered strictly mandatory. However, it shall anyway be considered as the state of the art: indirectly, its application to stand alone medical device software shall be considered as mandatory r anyway requested by notified bodies.
Let’s first discuss the field of application of the IEC 82304.
IEC 82304 is specifically related to the so-called health software, that is defined as “software intended to be used specifically for maintaining or improving health of individual persons, or the delivery of care”.
Whereas IEC 62304 is specifically related to medical device software, thus medical products with a well specific medical-related intended use, IEC 82304 deals with any kind of software. However, IEC 82304 deals with only stand-alone software, independently if this software are regulated as medical devices or not. In contrast, IEC 62304 includes any type of medical software, either standalone or embedded to a specific hardware device.
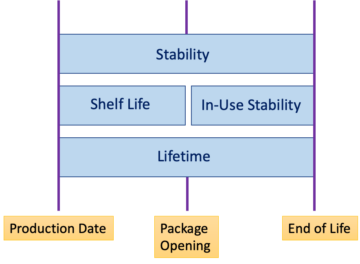
Software life cycle according to IEC 82304
In few words, we can say that IEC 82304 expands the requirements of IEC 62304. In fact, 62304 does not include the requirements for software validation, but only verification activities aimed at ensuring the device has been properly designed according to its intended function.
The validation activities are, in fact, specifically requested on IEC 82304, by introducing ad requirements for the Product Use Requirements, Product Validation Plans and Reports.
Product Use Requirements for Health Software
Section 4.2 of the IEC 82304 standard defines the type of use requirements it is necessary to specify for health software. In summary, these use requirements may be related to:
- requirements that address the intended use ;
- Interface / user interface requirements ;
- requirements for immunity from or susceptibility to unintended influence by other software using the same hardware resources
- privacy and security requirements
- requirements for accompanying documents such as instructions for use
- Requirements for support (Upgrades from previous versions, rollback to the previous version after upgrade , timely security patches and updates, decommisioning, irreversible deletion, transfer and/or retention of data, etc.
Health Software Product Validation
As we have been mentioned before, the validation requirements for stand-alone software were not included in the IEC 62304; however, these requirements are specifically requested in the framework of EC I82304. Obviously the validation plan shall address the user requirements related to the product.
Specifically, the following items shall be included in the validation plan:
- identify the validation scope and the corresponding validation activities
- identify the constraints that potentially limit the feasibility of validation activities
- select appropriate validation methods, input information, and associated acceptance criteria for successful validation
- Identify the enabling systems or services needed to support validation;
- Specify the required qualification of the validation personnel
- Define the appropriate level of independence of the validation team from the design team
The standard defines as well specific requirements related to the validation report.
Instructions for Use for Health Software
In the last section of IEC 82304, requirements related to instruction for use for health (stand-alone) software are described. We will not enter in details of all these requirements and we will briefly explain the most significant ones.
First, a specific section is related to the description of the health software. This description shall include the following elements:
- the intended use of the Health Software Product;
- a brief description of the Health Software Product, including the essential functions;
- any operational security options for the use of the Health Software;
- any known technical issues, limitations, disclaimer, or contraindication(s).
These requirements are very similar to the requirements specific requested by the EU MDR and IVDR Annex II, related to the preparation of technical documentation.
One last section that is relevant to discuss is related to the requirements to be mentioned in the IFU for the installation of the software. In this context, the following elements shall be considered:
- a statement whether the installation can be done by the user or shall be done by or with the assistance of the manufacturer;
- the system requirements for the software and hardware platforms intended to execute the Health Software;
- operational security options for the Health Software to be set at installation time;
- any critical dependencies on other applications;
- the configuration requirements;
- the system interface requirements;
- the details of the supported software platform;
- the installation instructions or a reference to where the installation instructions are to be found.
Subscribe to QualityMedDev Newsletter
QualityMedDev is an online platform focused on Quality & Regulatory topics for medical device business; Follow us on LinkedIn and Twitter to stay up to date with most important news on the Regulatory field.
QualityMedDev is one of the largest online platform supporting medical device business for regulatory compliance topics. We provide regulatory consulting services over a broad range of topics, from EU MDR & IVDR to ISO 13485, including risk management, biocompatibility, usability and software verification and validation and, in general, support in preparation of technical documentation for MDR.
Our sister platform QualityMedDev Academy provides the possibility to follow online and self-paced training courses focused on regulatory compliance topics for medical device. These training courses, developed in collaboration with highly skilled professionals in the medical device sector, allows you to exponentially increase your competencies over a broad range of quality and regulatory topics for medical device business operations.

Do not hesitate to subscribe to our Newsletter!
- SEO Powered Content & PR Distribution. Get Amplified Today.
- Platoblockchain. Web3 Metaverse Intelligence. Knowledge Amplified. Access Here.
- Source: https://www.qualitymeddev.com/2022/10/08/iec-82304/
- a
- Academy
- acceptance
- According
- accurate
- activities
- actually
- Ad
- address
- After
- All
- allows
- alone
- Although
- and
- Annex
- Application
- applications
- appropriate
- Art
- article
- Assistance
- associated
- before
- briefly
- broad
- business
- collaboration
- compliance
- Configuration
- considered
- constraints
- consulting
- context
- contrast
- Corresponding
- courses
- criteria
- critical
- data
- Date
- deal
- Deals
- Defines
- delivery
- described
- description
- Design
- designed
- details
- developed
- Development
- device
- Devices
- different
- discuss
- discussing
- documentation
- documents
- EC
- either
- elements
- embedded
- enabling
- ensuring
- Enter
- essential
- etc
- Ether (ETH)
- EU
- Europa
- execute
- expands
- Explain
- exponentially
- few
- field
- First
- focused
- follow
- following
- found
- Framework
- from
- function
- functions
- General
- Hardware
- hardware device
- Health
- highlighting
- highly
- However
- HTTPS
- immunity
- important
- improving
- in
- include
- included
- includes
- Including
- Increase
- independence
- independently
- indirectly
- individual
- influence
- information
- input
- instructions
- Interface
- introducing
- issues
- IT
- items
- Kind
- known
- largest
- Last
- Level
- Life
- LIMIT
- limitations
- LINK
- Look
- mailchimp
- management
- mandatory
- Manufacturer
- MDR
- medical
- medical device
- mentioned
- methods
- most
- Navigation
- necessary
- news
- ONE
- online
- operational
- Operations
- Options
- Other
- Patches
- persons
- plan
- plans
- platform
- Platforms
- plato
- Plato Data Intelligence
- PlatoData
- plugin
- possibility
- Posts
- potentially
- previous
- Product
- Products
- professionals
- properly
- protocols
- provide
- provides
- qualification
- quality
- range
- reach
- regulated
- regulatory
- Regulatory Compliance
- related
- relevant
- report
- Reports
- required
- Requirements
- retention
- Risk
- risk management
- same
- scope
- Section
- sector
- security
- Services
- set
- significant
- similar
- skilled
- Software
- software development
- specific
- specifically
- stand
- standalone
- standard
- standards
- State
- Statement
- stay
- subscribe
- successful
- such
- SUMMARY
- support
- Supported
- Supporting
- system
- Systems
- team
- Technical
- terms
- test
- The
- The State
- time
- to
- Topics
- Training
- transfer
- Updates
- upgrades
- URL
- us
- usability
- use
- User
- User Interface
- validation
- Verification
- version
- whether
- will
- WordPress
- WordPress plugin
- words
- Your
- zephyrnet