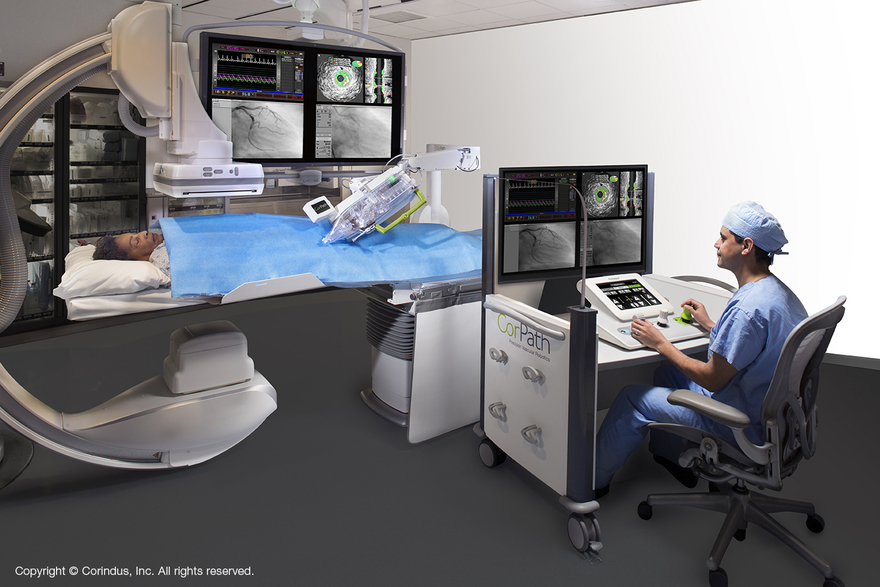
On May 16, 2023, the NMPA granted innovation approval to Corindus Inc., for its coronary interventional surgical control system accessories. It is the fifth imported innovative device approved by the NMPA so far in 2023, after Medtronic, Alcon, Ubiosis, and Conavi Medical’s approvals.
NMPA issued the review report for the device on May 22, 2023.
Product Description, Pre-clinical and Clinical
As NMPA standardizes and streamlines the review process for fast-track approval, it is vital for manufacturers to comply with requirements of following items if you have similar equipment entering China market.
Product overview
- Product structure and composition
- Scope of product application
This product is used in healthcare facilities in conjunction with the Company’s CorPath GRX System to assist clinicians in delivering and operating guidewires, guide tubes, and rapid exchange balloon dilation catheters/stents during percutaneous coronary interventions. Before the use of the product, a qualified clinician should judge the severity and complexity of the patient according to the current diagnosis and treatment guidelines, and for high-risk patients or complex lesions, the clinician should strictly follow the preoperative evaluation to judge whether it is applicable. It’s not for emergency use.
- Model/Specification
- Working principle
This product is a disposable passive accessory for coronary interventional surgical control system, including two components, cassette and drive gear, which are made of polymer materials. The cassette shell is made of polycarbonate, and the internal components are mainly made of acetal, polyurethane and polycarbonate; The drive gears are made of polycarbonate and acetal. When used, it needs to be installed at the end of the manipulator arm of the control system, and the controlled interventioAn instruments such as guidewires and catheters are installed in a specific area of the cassette belt, and the drive components in the cassette are driven by the control system to promote the controlled instruments to achieve different movements.
Pre-clinical
- Product performance research
- Biocompatibility
The applicant evaluated the biocompatibility of the finished product according to GB/T 16886.1-2011. The evaluated materials were exposed to the human circulating blood circuit for a short period of time, and biological tests (cytotoxicity, intradermal reaction, sensitization, acute systemic toxicity, hemolysis, pyrogen) were carried out. Among them, the report of cassette part was issued by the overseas testing institute, and that of gear part was issued by the domestic testing center.
- Disinfection
- Packaging and shelf life
- Animal study
Clinical Evaluation
The applicant used overseas clinical data to support the clinical evaluation.
- Precision GRX trial:
It was carried out in 20 clinical sites, using a prospective, single-group target design. A total of 980 subjects enrolled, 980 subjects with a total of 1233 lesions, according to the definition of AHA/ACC lesion classification, of which the proportion of subjects with type B2 lesion was 23.3%, the proportion of subjects with type C lesion was 45.5%, and that with CTO (chronic total occlusive lesion) was 10.2%.
- Precision GRX PMS trial:
It was carried out in 8 clinical sites, using a prospective, single-group target design. A total of 239 subjects were enrolled. The proportion of participants with B2/C (complex) lesions in the ACC/AHA classification was 70.2%, and that with CTO lesions was 2.1%.
For more information on the two clinical trials, please email info@ChinaMedDevice.com.
Risk-Benefit analysis
NMPA concludes that benefits brought to applicable population outweigh the risks.
Possible adverse events include instrument failure, PCI surgery-related adverse events, etc., and the main risk is patient injury caused by loss of exercise control. After analysis, device failure does not significantly increase the patient’s radiation dose exposure, delay treatment, etc., the total operation time may be prolonged, and the risk of loss of motor control is relatively acceptable.
The use of this product reduces radiation exposure during surgery and is estimated to improve physician attention and operation performance. The product can realize quantitative size measurement, assist physicians to judge lesion length, and based on literature data, it can reduce the incidence of incomplete vascular lesion coverage compared with traditional methods. The standardized operation mode makes the instrument delivery more stable, which can theoretically reduce the complications caused by the operation differences.
Please email us at info@ChinaMedDevice.com to see if NMPA released review reports for your device. We can translate for you with nominal fees.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- EVM Finance. Unified Interface for Decentralized Finance. Access Here.
- Quantum Media Group. IR/PR Amplified. Access Here.
- PlatoAiStream. Web3 Data Intelligence. Knowledge Amplified. Access Here.
- Source: https://chinameddevice.com/coronary-surgical-control-system/
- :is
- :not
- 1
- 10
- 16
- 2%
- 20
- 2023
- 22
- 23
- 70
- 8
- a
- acceptable
- accessories
- accessory
- According
- Achieve
- adverse
- After
- among
- analysis
- and
- applicable
- approval
- approvals
- approved
- ARE
- AREA
- ARM
- AS
- assist
- At
- attention
- based
- BE
- before
- benefits
- blood
- brought
- by
- CAN
- carried
- caused
- Center
- China
- circulating
- classification
- Clinical
- clinical trials
- clinicians
- COM
- Company’s
- compared
- complex
- complexity
- comply
- components
- conjunction
- control
- controlled
- coverage
- CTO
- Current
- data
- delay
- delivering
- delivery
- description
- Design
- device
- diagnosis
- differences
- different
- does
- Domestic
- dose
- drive
- driven
- during
- emergency
- end
- enrolled
- entering
- equipment
- estimated
- etc
- evaluated
- evaluation
- events
- exchange
- Exercise
- exposed
- Exposure
- facilities
- Failure
- far
- Fees
- fifth
- follow
- following
- For
- Gear
- gears
- granted
- guide
- guidelines
- Have
- healthcare
- high-risk
- HTTPS
- human
- if
- improve
- in
- Inc.
- incidence
- include
- Including
- Increase
- information
- Innovation
- innovative
- installed
- Institute
- instrument
- instruments
- internal
- Issued
- IT
- items
- ITS
- jpg
- judge
- Length
- literature
- loss
- made
- Main
- mainly
- MAKES
- Manufacturers
- Market
- materials
- May..
- measurement
- methods
- Mode
- more
- Motor
- movements
- needs
- of
- on
- operating
- operation
- or
- out
- outweigh
- overseas
- part
- participants
- passive
- patient
- patients
- performance
- period
- physician
- plato
- Plato Data Intelligence
- PlatoData
- please
- PMS
- polymer
- population
- process
- Product
- promote
- proportion
- prospective
- qualified
- quantitative
- Radiation
- rapid
- reaction
- realize
- reduce
- reduces
- relatively
- released
- report
- Reports
- Requirements
- review
- Risk
- risks
- see
- Shelf
- Shell
- Short
- should
- significantly
- similar
- Sites
- Size
- So
- so Far
- specific
- stable
- structure
- such
- support
- Surgery
- surgical
- system
- systemic
- Target
- Testing
- tests
- that
- The
- Them
- this
- time
- to
- Total
- traditional
- translate
- treatment
- trial
- trials
- two
- type
- us
- use
- used
- using
- vital
- was
- we
- were
- when
- whether
- which
- with
- you
- Your
- zephyrnet