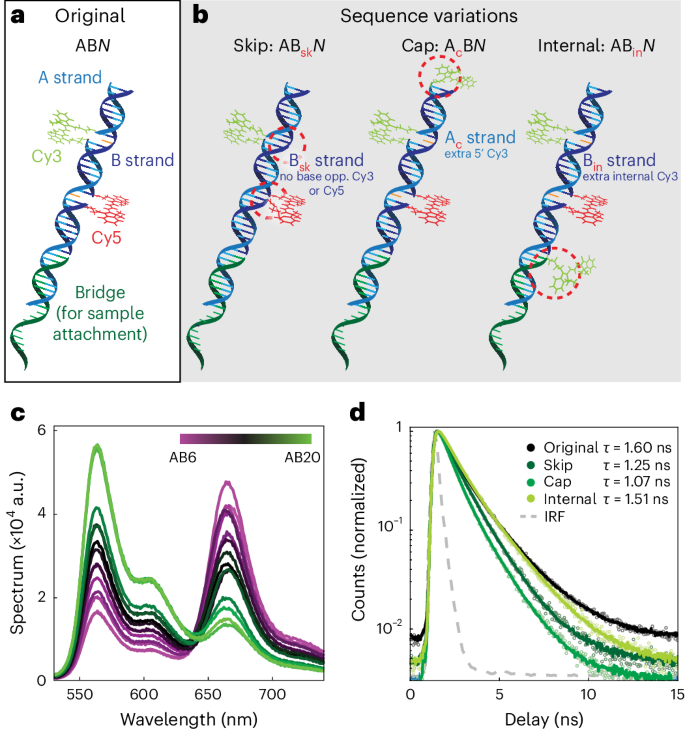
Design of FRETfluor labels
To engineer a collection of fluorescent labels with unique spectroscopic signals, high chemical homogeneity and minimal structural complexity, we utilized a simple set of biomolecular building blocks: DNA oligomers functionalized with either Cy3 or Cy5 dye. We chose phosphoramidite incorporation of dyes into the DNA backbone to limit dipole rotational mobility29 and to improve photostability38. We first created a series of ‘ABN’ constructs incorporating non-sulfonated Cy3 and Cy5 into the ‘A’ and ‘B’ DNA strands, respectively, separated by N base pairs where 6 ≤ N ≤ 20 (Fig. 1a shows AB9). An additional ‘bridge’ strand enables the sequence-specific labelling of nucleic acid targets or the addition of functional groups for common labelling chemistries. FRETfluor constructs are similar in size to a fluorescent protein (~30 kDa) but with a higher aspect ratio. The single-exponential-fitted lifetime and background-subtracted brightness of the Cy3 donor in AB0 (lacking Cy5) were measured to be τAB0 = 1.60 ± 0.03 ns and 0.31 ± 0.01 counts ms–1 μW–1, respectively. Supplementary Table 1 lists the FRETfluor oligo sequences.
a, FRETfluor design for ABN constructs (A strand, cyan; B strand, blue; unpaired bases, orange), shown here with a bridge (green) for the sequence-specific labelling of nucleic acids. b, FRETfluor design variations ABsk, AcB and ABin, used to create additional unique spectroscopic signatures. Key changes for each construct are highlighted with a dotted red circle. c, Bulk emission spectra of ABN constructs demonstrate that FRET tunes the emission, as expected. d, Fluorescence lifetime measurements of aggregated single-molecule data show that the Cy3 lifetime depends on the local DNA sequence and attachment chemistry (single-exponential fits are shown in solid lines; data, open circles; IRF, grey dotted line).
We expected that the spectroscopic emission of different ABN FRETfluors would follow a smooth manifold in the detection parameter space (brightness, lifetime, emission spectrum or FRET efficiency), similar to previous FRET studies using DNA scaffolds19,25. By modifying the DNA sequence and attachment chemistry of the Cy3 donor or by including an additional Cy3, we created additional FRETfluor types with different photophysical properties from ABN (Fig. 1b). ‘Skip’ oligos, Bsk, lack the unpaired bases opposing Cy3 and Cy5, lowering the lifetime and quantum efficiency of Cy3 compared with ABN constructs (τABsk0 = 1.25 ns ± 0.03; green brightness, 0.26 ± 0.01 counts ms–1 μW–1). ‘Cap’ oligos, Ac, carry an additional single-tethered Cy3 at the 5’ end, increasing the brightness and lowering the net Cy3 lifetime (τAcB0 = 1.07 ns ± 0.03; green brightness, 0.40 ± 0.01 counts ms–1 μW–1). ‘Internal’ oligos, Bin, incorporate an additional Cy3 between the 3’ end of the bridge strand and the 5’ end of the B strand, increasing the brightness but only slightly affecting the net Cy3 lifetime (τABin0 = 1.51 ns ± 0.03; green brightness, 0.560 ± 0.015 counts ms–1 μW–1) (Supplementary Note 1, Supplementary Table 2 and Supplementary Fig. 1).
In bulk measurements, we observed the expected decrease in FRET efficiency for FRETfluors with increasing N (Fig. 1c). Single-exponent fits to the measured lifetime decays of Cy3 for each type of construct illustrate the effect of local sequence and attachment chemistry on donor lifetime (Fig. 1d). In total, 41 FRETfluor constructs were synthesized: 15 of ABN, 8 of ABskN, 9 of AcBN and 9 of ABinN.
Detection of FRETfluor labels in the ABEL trap
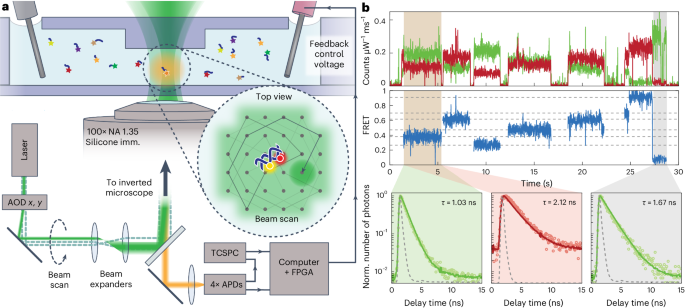
To spectroscopically characterize the single-molecule emission of each FRETfluor, we employed a custom-built ABEL trap (Fig. 2a). Originally developed by Cohen and Moerner37 to overcome common technical challenges in single-molecule measurements, the ABEL trap uses closed-loop feedback to electrophoretically counteract the effects of Brownian motion on single molecules in a solution-phase environment39,40. Critically, ABEL traps enable precise spectroscopic characterization of single molecules across multiple parameters, including brightness, fluorescence lifetime, anisotropy and emission spectrum41,42,43. With the record of applied voltages, the pattern of movement for a particle in the trap can be extrapolated and used to estimate hydrodynamic properties, including diffusion coefficient and electrophoretic mobility, allowing us to monitor the size of a FRETfluor or FRETfluor-labelled molecule44.
a, Schematic of ABEL trap detection: FRETfluors (blue DNA; coloured stars) are detected in a microfluidic cell atop an inverted microscope. Here 532 nm pulsed laser excitation is scanned across the field of view using x and y acousto-optic deflectors (AODs). A FRETfluor in the trapping region fluoresces when it is co-localized at the scanned laser position, enabling closed-loop feedback control over its position via electrodes that apply x and y voltages to electrokinetically move the particle back to the trap centre. Spectroscopic data are simultaneously acquired (time-correlated single-photon counting (TCSPC); 4× APDs for polarization and red/green channels). FPGA, field-programmable gate array; NA, numerical aperture. b, Raw ABEL trap data showing signals from seven different FRETfluors over 30 s (10 ms binning). Background-subtracted brightness in the red and green channels increases during trapping events (top). FRET efficiency calculated from the red and green brightness traces (middle). The grey dotted lines indicate the expected FRET values for each class of FRETfluor. Fluorescence lifetime decays for the green and red channels during the first trapping event (brown background above; split into green and red backgrounds) versus donor lifetime when the acceptor is blinking or photobleached (grey background) (bottom). The IRF is shown by the grey dotted line.
Raw ABEL trap data reveal different FRET values for FRETfluors in a mixture (Fig. 2b). Lifetime fitting confirms that the observed Cy3 lifetime is substantially shortened by FRET with Cy5 (Fig. 2b, bottom left) relative to the Cy3 lifetime after Cy5 photobleaches (Fig. 2b, bottom right). Supplementary Fig. 2 shows the annotated raw trapping data for a mixture of FRETfluors, with additional discussion in Supplementary Note 2.
Typical trapping throughput for FRETfluors in our ABEL trap setup is ~0.1 molecules s–1 pM–1; therefore, a measurement time of ~15 min is sufficient to analyse FRETfluor-labelled samples at concentrations down to tens of femtomolar (Supplementary Fig. 3 and Supplementary Note 3). The ABEL trap’s high sensitivity to ultralow concentrations of FRETfluors is a major advantage of our approach.
Multi-parameter characterization of FRETfluor emission
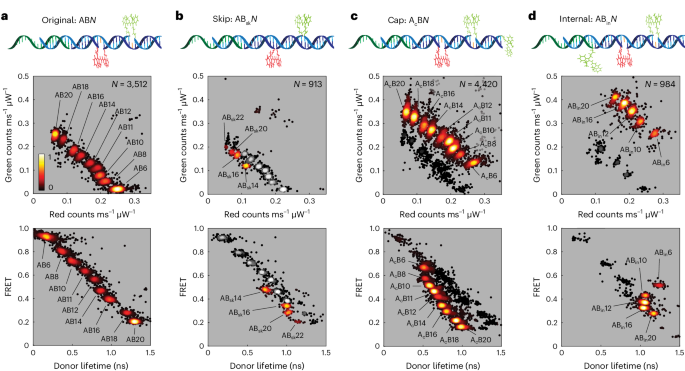
The measured red and green channel brightness, donor lifetime and FRET efficiency data produce tight clusters for each type of FRETfluor construct (Fig. 3), indicating that these parameters can be used to classify single FRETfluors. Cluster size depends on the duration and photon content averaged into each data point; here we included trapping levels with durations of >150 ms. Under our experimental conditions, these levels average more than 5,000 photons each (Supplementary Fig. 4).
The red–green and lifetime–FRET projections of the level-by-level data from trapped FRETfluor constructs show clusters in different regions of the measured multi-parameter space. Each point represents the average value of data from one level with duration of >150 ms; the number of levels passing this filter for each dataset is shown as N at the top-right corner of each red–green projection. The black–red–yellow heat map denotes the relative scatter-plot density from low (black) to high (yellow). a, Set of nine ABN constructs showing distinct clusters in both red–green (top) and lifetime–FRET projections (bottom). b, Data for four ABskN constructs were taken along with nine ABN constructs (grey scale) to verify the shifted cluster locations. c,d, Data for nine AcBN constructs (c) and five ABinN constructs (d) similarly show distinct clusters that are distinguishable from the original ABN construct locations.
We tested FRETfluors individually and in various combinations to determine the characteristic emission properties and cluster widths for each of the 41 FRETfluor constructs (Supplementary Table 3). As expected, stepwise changes in donor–acceptor spacing within a FRETfluor construct type show correlated changes in the cluster position. For example, for a mixture of nine different ABN labels, red and green brightnesses are inversely correlated (Fig. 3a, top), and the donor lifetime is inversely correlated with the FRET efficiency (Fig. 3a, bottom).
Changes to the donor photophysics shift the FRETfluor clusters to other regions of the detection parameter space: four out of the eight ABskN constructs, nine AcBN constructs and five ABinN constructs showed unique signals relative to the ABN constructs and one another (Fig. 3b–d). Constructs not included in Fig. 3 were determined to statistically overlap with one or more clusters beyond a 2.5% misclassification threshold (see the ‘Robust classification for mixtures of FRETfluors’ section) and therefore are not included here (Supplementary Figs. 5–7).
These results show that relatively small changes in dye photophysics, such as the 15–20% difference in donor brightness and lifetime between ABskN and ABN FRETfluors, can produce uniquely identifiable spectroscopic signatures. We observed that donor brightness and lifetime changes were not perfectly correlated, confirming that both radiative and non-radiative lifetimes are influenced by the physicochemical environment41. Such tuning is useful for FRETfluor design; Supplementary Notes 4 and 5 and Supplementary Fig. 8 detail simulations showing that decoupled donor lifetime and brightness changes produce nearly orthogonal shifts in a FRET curve within the multi-parameter detection space.
To determine whether changing environmental conditions would substantially impact FRETfluor performance, we tested salt and pH across a physiological range (0 to 150 mM NaCl, pH 6.5 to 8.5). FRETfluor signals were consistent across all pH values tested and exhibited small (~10%) salt-induced reductions in the brightness of ABinN-type, ABN-type and AcBN-type constructs and in the donor lifetime of ABinN-type constructs (Supplementary Fig. 9 and Supplementary Note 6). These small shifts might be amenable to calibration such that FRETfluors could report on the local physicochemical environment with well-separated relative cluster locations so that each FRETfluor remains uniquely identifiable.
Robust classification for mixtures of FRETfluors
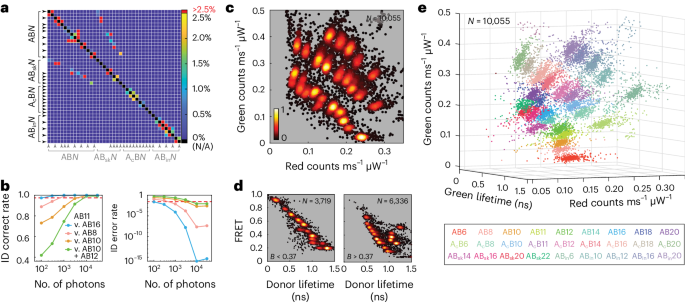
In a mixture of FRETfluors, reliable classification of single molecules depends on experimental factors (such as measurement duration and precision) as well as on the set of FRETfluors used. We analysed all the possible pairwise combinations of our 41 FRETfluor constructs to determine which pairs presented higher chances of mutual misclassification. Each cluster of levels from the ABEL trap data was fit as a three-dimensional (3D) Gaussian distribution (red and green brightnesses and green lifetime), and one-tail integrations were performed over the parameter space to generate a confusion matrix (Fig. 4a). We set a 2.5% misclassification threshold for identifying unfavourable FRETfluor combinations. The maximum pairwise misidentification probability was found to be ~30% for ABsk10 and AB8 (Supplementary Fig. 10). Under 0.7% (33 of the 1,640 pairs) exceeded our 2.5% threshold, and the likelihood of misclassification for most pairs is vanishingly small (Supplementary Fig. 11). On the basis of this analysis, we identified a subset of 27 FRETfluors suitable for use in a single mixture, indicated by the arrows in Fig. 4a (bold typeface in Supplementary Table 3; Supplementary Fig. 12 shows the misclassification matrix).
a, Misclassification probability calculated as a one-tailed Gaussian overlap for each pair of FRETfluors. True cluster identity (left) and incorrect cluster identity (bottom) intersect at squares coloured according to the probability of misidentification for each combination. The colour bar shows the probability of misclassification for each pair, capped here at 2.5% (red). Self-identification (black on-diagonal) is variable depending on the set of FRETfluors selected for use. b, Correct (left) and incorrect (right) identification rates for FRETfluor AB11 as a function of the number of photons used for analysis when considering confusion with either AB16 (blue), AB8 (pink), AB10 (orange) or both AB10 and AB12 (green). The cutoff of 97.5% is shown as a red dotted line. c, Red–green projection of data from multiplexed detection of 27 FRETfluors in a single sample. d, Two brightness slices of the same dataset shown in a lifetime–FRET projection, separated at B = 0.37 counts ms–1 μW–1. e, 3D projection of the same dataset coloured according to cluster membership for each of 27 FRETfluor labels per the key (bottom). Supplementary Video 1 shows a rotating view of this plot. In c–e, each point represents the average value of data from one level with duration of >150 ms; the number of levels passing this filter for each dataset is shown as N at top-right corner of each panel.
In this work, we did not attempt to optimize the measurement throughput: FRETfluors were trapped until photobleaching or until their natural exit from the trap, usually after several seconds of measurement. To determine whether similar levels of discriminative power might be achieved with a higher throughput by limiting the trapping time, we examined the effect of the number of photons per point on the spread of the FRETfluor clusters (Supplementary Note 7). Here the typical total photon arrival rates are >25 kHz, and the levels used for analysis are typically based on several thousand photons. Limiting the number of photons per data point broadens the clusters and increases misclassification (Fig. 4b shows the case of a typical FRETfluor, AB11). For ~100 photons per point, AB11 can be correctly identified compared with its nearest neighbours, AB10 and AB12, with only about 45% (two-tailed) and 75% (one-tailed) accuracy. However, AB11 can still be readily differentiated from more distant clusters (AB8, 90.0% accuracy; AB16, 97.5% accuracy). At 3,000 photons per point, identification is at worst 92% (two-tailed nearest neighbours). Correct identification of AB11 from AB10 and AB12 surpasses 97.5% accuracy at ~6,000 photons per point. Thus, the throughput of FRETfluor identification could be improved by sampling each construct for long enough to gather ~104 photons above the background, which requires only ~400 ms per trapping event (neglecting blinking effects).
We next tested our ability to experimentally distinguish this optimized FRETfluor set. We combined the complete set of 27 FRETfluors in a dilute sample mixture (~2 pM total; ~75 fM of each FRETfluor). Figure 4c shows a red–green projection illustrating clear separation of clusters; these clusters can be further differentiated in lifetime–FRET projections (Fig. 4d), here showing two brightness cuts above and below a brightness threshold of 0.37 counts ms–1 μW–1. A 3D view with each cluster coloured according to its most likely identity shows the distribution of cluster positions within the detection parameter space (Fig. 4e and Supplementary Video 1). The raw data in Supplementary Fig. 2 show this combination of FRETfluors and are annotated with tag identities. Cluster locations are consistent with the expected values (Supplementary Fig. 13).
Wash-free labelling of biomolecular targets
The specificity of FRETfluors for biomolecular targets can be programmed using common attachment chemistries on the bridge strand for conjugation to, for example, antibodies, specific chemical linkers or nucleic acid sequences.
We first targeted FRETfluors to specific nucleic acids via sequence complementarity between a bridge strand and RNA and DNA targets (Fig. 5a,b). Labelling specificity was confirmed by bulk electrophoretic mobility shift assay (EMSA) comparing FRETfluors equipped with either on- or off-target bridge sequences binding to an ssDNA target strand (Supplementary Fig. 14). We next tested RNA binding by designing bridge sequences complementary to regions predicted with high confidence to be part of a loop in the secondary structure of three mRNAs: enhanced green fluorescent protein mRNA (EGFP; 996 nt), firefly luciferase mRNA (FLuc; 1,929 nt) and ovalbumin mRNA (OVA; 1,438 nt)45. EMSA confirmed that off-target bridge sequences did not bind mRNA, whereas on-target sequences hybridized with each mRNA tested (Supplementary Note 8 and Supplementary Fig. 15). We similarly confirmed by EMSA that a FRETfluor with an on-target bridge sequence could invade and bind near the end of a dsDNA reverse transcription polymerase chain reaction (RT-PCR) product (Supplementary Fig. 16).
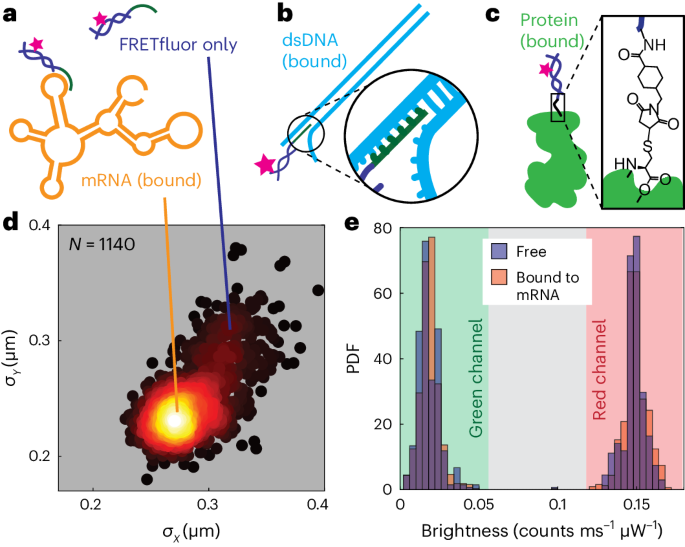
a, FRETfluor tag (dark blue and pink) not bound to any target (top) and the same FRETfluor targeted to a loop on mRNA (bottom) via complementarity of the bridge strand (green). b, FRETfluor tag (dark blue and pink) targeted to dsDNA (cyan) via base complementarity of a bridge strand (green). c, Illustration of FRETfluor site-specifically labelling a protein (light green) via a maleimide–NHS ester bifunctional linker. d, Scatter plot of standard deviation of position in x and y for trapped molecules of AB6 FRETfluor targeted to FLuc mRNA. Points are coloured according to the local relative scatter-plot density; each point is calculated from localization trajectories based on 1,000 photons; N = 1,140 points are shown in the scatter plot. e, Normalized histograms showing the probability distribution functions of green (left) and red (right) signals for AB6 on FLuc mRNA are nearly identical for the free (blue histogram) and mRNA-bound (orange histogram) FRETfluor.
To site-specifically label proteins with FRETfluors, we utilized a bifunctional linker containing both an N-hydroxysuccinimide (NHS) ester group and a maleimide group, which we reacted with a primary amine on a FRETfluor and a cysteine sulfhydryl on the target protein (Fig. 5c). We verified covalent labelling by native polyacrylamide gel electrophoresis for two target proteins, poly-A binding protein (Pab1) and a Class A J-domain protein (Ydj1) from Saccharomyces cerevisiae, each mutated to have a single accessible cysteine (Supplementary Fig. 17).
Fluorescence labelling protocols usually require substantial washing or sample purification. In an ABEL trap, however, labelled biomolecules such as mRNA can be readily distinguished from free labels by estimating the transport properties of each trapped object. Objects with a larger hydrodynamic radius diffuse more slowly than free labels due to their size, leading to tighter confinement around the trap’s centre. We observed that the distribution of standard deviations in position from the trap centre in each direction, σx and σy, for FRETfluors bound to a target mRNA exhibits two distinct clusters (Fig. 5d). We attribute the more tightly confined state to the correctly labelled FRETfluor–mRNA complex, whereas the less confined cluster is consistent with a trapped FRETfluor without the target present. These clusters can be used to separate labelled target molecules from free FRETfluors in post hoc analyses for wash-free labelling and detection of diverse biomolecular targets. In principle, sample throughput could also be increased by using this information on the fly to quickly reject free FRETfluors from the trap.
Cyanine dyes are well known to be sensitive to their environment in contexts beyond DNA attachment, for example, via protein-induced fluorescence enhancement46,47 or solution and local environment composition27,34. Here protein-induced fluorescence enhancement was neither observed on FRETfluor attachment to proteins nor were the FRETfluor signals altered by binding to mRNA or DNA (Fig. 5e). Together with the limited effects of salt and pH previously discussed, these results suggest that FRETfluor structure and attachment chemistry are dominant environmental influences on cyanine dye photophysics in this study, and that the FRETfluor structure may partially protect the cyanine dyes against interactions with the target molecules or solvent. Future use of non-isomerizing48 or otherwise photostabilized49 cyanine dyes could further protect FRETfluors from environmental effects.
Detecting low-abundance targets in biomolecular mixtures
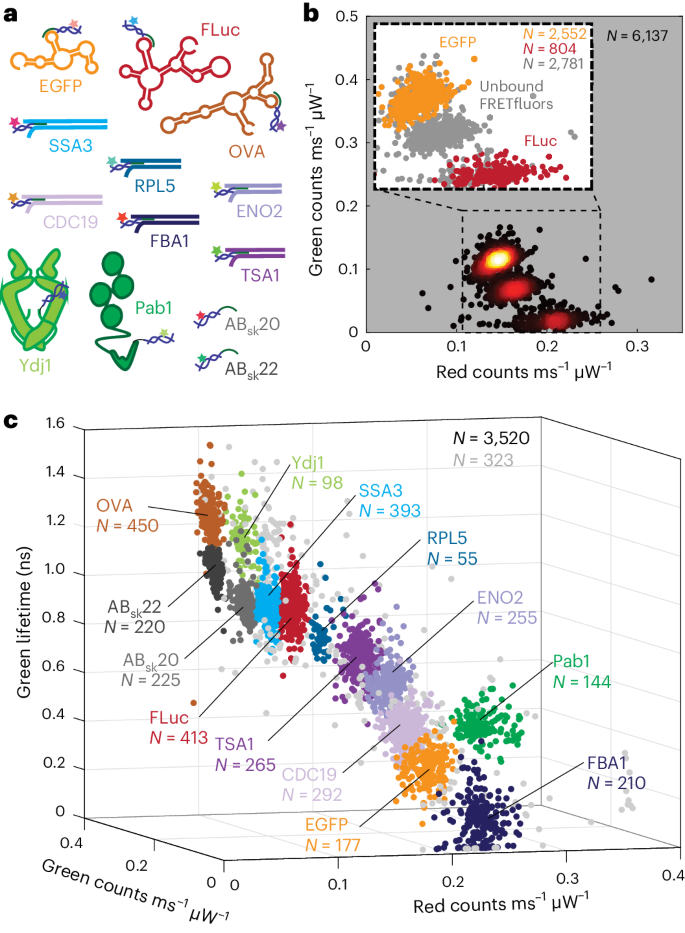
To explore the suitability of FRETfluors for multiplexed detection of low-abundance biomarkers, we tested both simple and complex mixtures of FRETfluor-labelled mRNA, dsDNA and proteins (Fig. 6a). We first performed wash-free labelling and readout of a 1:1 mixture of FLuc and EGFP mRNAs labelled with FRETfluors AB6 and AB12, respectively. We also included an off-target-free FRETfluor, AB10, to control non-specific labelling. We observed three distinct spectroscopic populations corresponding to AB6, AB10 and AB12 (Fig. 6b). Separating bound and unbound populations in each cluster revealed that both AB6 and AB12 bind to their target mRNA with 71% and 73% binding efficiency, respectively, whereas all AB12 was free, confirming that off-target labelling or cross-reactivity did not occur and that FRETfluor spectroscopic signatures are unchanged by target binding (Fig. 6b (inset) and Supplementary Fig. 18).
a, Identities of all mRNA (3), dsDNA (6), proteins (2) and target-less FRETfluor (2) samples. b, Scatter heat map of a red–green projection of data for a simple mixture of EGFP + AB12, FLuc + AB6 and AB10 (no target). Points are coloured according to the local relative scatter-plot density. The inset shows the scatter plot of signals from unbound FRETfluors of all types (grey) and bound EGFP + AB12 (orange) and FLuc + AB6 (red). Each point in the main panel and the inset represents 1,000 photons, with ‘bound’ and ‘unbound’ assignments based on localization trajectories and red–green brightness taken from the corresponding level with duration of >100 ms; N = 6,137 total points. c, 3D projection of spectroscopic data for a complex mixture of FRETfluor-labelled mRNA, dsDNA and proteins (N = 3,520 points). Cluster colours are assigned as per a (N indicated for each cluster in the corresponding colour), with unassigned data points shown in light grey (N = 323 points). Each point represents the average value of data from one level with duration of >150 ms. Supplementary Fig. 18 provides the red–green and lifetime–FRET projections of this dataset.
Finally, we tested multiplexed detection of low-abundance biomarkers in a more complex mixture, as might be found in biomedical or environmental samples. We separately labelled mRNAs (EGFP, FLuc and OVA), proteins (Pab1 and Ydj1) and dsDNA fragments produced by RT-PCR from abundantly expressed and stress-response-related transcripts in S. cerevisiae50 (FBA1, CDC19, ENO2, TSA1, RPL5 and SSA3), with a subset of the 27-FRETfluor combination tested above (Supplementary Tables 4 and 5). Together with two off-target FRETfluors as controls, the analysis of a low-concentration mixture of all the labelled targets (~350 fM each) shows all the FRETfluors present with their expected spectroscopic signatures (Fig. 6c); clusters do not shift on binding the targets. A closer examination of each cluster (Supplementary Fig. 19) shows that bound and unbound FRETfluors can be differentiated as expected, and that FRETfluors do not cross-react to label incorrect targets.
We found that cluster occupancy was approximately—but not exactly—reflective of the mixture stoichiometry. Discrepancies may arise from complicating factors such as trapping bias or dye photophysics, which could influence both number and proportion of the observed levels meeting the analysis filter criteria. We expect that these effects could, in principle, be calibrated out for each sample and label combination by comparison with known standards.
- SEO Powered Content & PR Distribution. Get Amplified Today.
- PlatoData.Network Vertical Generative Ai. Empower Yourself. Access Here.
- PlatoAiStream. Web3 Intelligence. Knowledge Amplified. Access Here.
- PlatoESG. Carbon, CleanTech, Energy, Environment, Solar, Waste Management. Access Here.
- PlatoHealth. Biotech and Clinical Trials Intelligence. Access Here.
- Source: https://www.nature.com/articles/s41565-024-01672-8
- :is
- :not
- :where
- ][p
- 000
- 01
- 07
- 1
- 10
- 100
- 11
- 114
- 118
- 14
- 140
- 15%
- 150
- 16
- 19
- 1981
- 2%
- 20
- 2006
- 2008
- 2010
- 2011
- 2012
- 2014
- 2015
- 2016
- 2017
- 2021
- 25
- 26
- 27
- 29
- 30
- 31
- 33
- 34
- 35%
- 37
- 39
- 3d
- 3D view
- 4
- 40
- 41
- 42
- 438
- 45
- 46
- 48
- 49
- 5
- 50
- 51
- 6
- 60
- 8
- 9
- 90
- 97
- a
- ability
- About
- above
- accessible
- According
- Accounting
- accuracy
- achieved
- acquired
- across
- addition
- Additional
- ADvantage
- affecting
- After
- against
- aggregated
- AL
- All
- Allowing
- along
- also
- altered
- altman
- amenable
- Amplified
- an
- analyse
- analyses
- analysis
- Anchor
- and
- Another
- Antibodies
- any
- Application
- applied
- Apply
- approach
- ARE
- arise
- around
- Array
- arrival
- AS
- aspect
- assigned
- assignments
- At
- attached
- attempt
- average
- b
- back
- Backbone
- background
- backgrounds
- bar
- base
- based
- basis
- BE
- below
- between
- Beyond
- bias
- bind
- binding
- biomarkers
- biomedical
- Black
- Blocks
- Blue
- bold
- both
- Bottom
- bound
- BRIDGE
- broadens
- brown
- Building
- bulk
- but
- button
- by
- calculated
- CAN
- capped
- carry
- case
- cell
- centre
- chain
- challenges
- chances
- Changes
- changing
- Channel
- channels
- characteristic
- characterize
- chemical
- chemistry
- chose
- Circle
- circles
- class
- classification
- Classify
- clear
- click
- closer
- Cluster
- cohen
- collection
- combination
- combinations
- combined
- Common
- compared
- comparing
- comparison
- complementary
- complete
- complex
- complexity
- concentration
- concept
- conditions
- confidence
- CONFIRMED
- confirming
- confusion
- considering
- consistent
- construct
- constructs
- containing
- content
- contexts
- control
- controls
- cooper
- Corner
- correct
- correctly
- correlated
- Corresponding
- could
- counteract
- counting
- counts
- COVALENT
- create
- created
- criteria
- curve
- Custom-built
- cuts
- cyan
- Dark
- data
- data points
- decoupled
- decrease
- demonstrate
- denotes
- density
- dependence
- dependent
- Depending
- depends
- Derivatives
- Design
- designing
- detail
- detect
- detected
- Detection
- Determine
- determined
- developed
- deviation
- DID
- difference
- different
- differentiated
- Diffusion
- direct
- direction
- discussed
- discussion
- distance
- Distant
- distinct
- distinguish
- Distinguished
- distribution
- diverse
- dna
- do
- dominant
- doubly
- down
- due
- duration
- durations
- during
- dye
- e
- E&T
- each
- effect
- effects
- efficiency
- eight
- either
- emission
- employed
- enable
- enables
- enabling
- end
- energy
- engineer
- enhanced
- enhancement
- enough
- Environment
- environmental
- environments
- equipped
- estimate
- Ether (ETH)
- Event
- events
- examination
- examined
- example
- exceeded
- exhibited
- exhibits
- Exit
- expect
- expected
- experimental
- experiments
- explore
- expressed
- factors
- false
- feedback
- field
- Fields
- Fig
- Figure
- filter
- First
- fit
- fits
- fitting
- five
- follow
- For
- found
- four
- fpga
- Free
- from
- full
- function
- functional
- functions
- further
- future
- gate
- gather
- generate
- greatly
- Green
- Group
- Group’s
- Have
- here
- High
- higher
- Highlighted
- However
- HTTPS
- i
- identical
- identifiable
- Identification
- identified
- identifying
- identities
- Identity
- illustrate
- illustrating
- image
- Impact
- improve
- improved
- improving
- in
- included
- Including
- incorporate
- incorporating
- incorrect
- increased
- Increases
- increasing
- indicate
- indicated
- indicating
- individual
- Individually
- influence
- influenced
- information
- integrations
- interactions
- internally
- intersect
- into
- Invade
- IT
- ITS
- KDA
- Key
- Kim
- known
- Label
- labelling
- Labels
- Lack
- lacking
- largely
- larger
- laser
- leading
- Lee
- left
- less
- Level
- levels
- lifetime
- light
- likelihood
- likely
- LIMIT
- Limited
- limiting
- Line
- lines
- LINK
- linked
- linker
- Lists
- local
- Localization
- locations
- Long
- Low
- lowering
- Main
- major
- map
- Marcus
- material
- Matrix
- maximum
- May..
- measured
- measurement
- measurements
- meeting
- membership
- methods
- Microscope
- Middle
- might
- min
- minimal
- mixture
- mobility
- molecular
- molecule
- Monitor
- more
- most
- motion
- motions
- move
- movement
- mRNA
- MS
- multiple
- mutual
- nanotechnology
- native
- Natural
- Nature
- Near
- nearly
- neglecting
- Neither
- net
- next
- NHS
- nine
- no
- Noise
- nor
- note
- Notes
- nt
- number
- numerical
- object
- objects
- observed
- occupancy
- occur
- of
- on
- ONE
- only
- open
- Optimize
- optimized
- or
- Orange
- original
- originally
- Other
- otherwise
- our
- out
- over
- Overcome
- overlap
- pair
- pairs
- panel
- parameter
- parameters
- part
- particle
- Passing
- Pattern
- per
- perfectly
- performance
- performed
- Photons
- pink
- plato
- Plato Data Intelligence
- PlatoData
- plot
- pm
- Point
- points
- populations
- position
- positions
- possible
- Post
- power
- precise
- Precision
- predicted
- present
- presented
- previous
- previously
- primary
- principle
- probability
- PROC
- processes
- produce
- Produced
- Product
- profiling
- programmed
- Projection
- projections
- properties
- proportion
- protect
- Protein
- Proteins
- protocols
- provides
- Quantum
- quickly
- R
- range
- Rate
- Rates
- ratio
- Raw
- raw data
- reacted
- reaction
- readily
- readout
- record
- Red
- reductions
- reference
- region
- regions
- reject
- relative
- relatively
- relaxation
- reliable
- remains
- report
- represents
- require
- requires
- research
- resonance
- Results
- reveal
- Revealed
- Reveals
- reverse
- right
- RNA
- rulers
- s
- salt
- same
- sample
- Scale
- SCI
- secondary
- seconds
- Section
- see
- selected
- selection
- sensitive
- Sensitivity
- separate
- separately
- separating
- Sequence
- Series
- server
- set
- setup
- seven
- several
- shift
- shifted
- Shifts
- Short
- shortened
- show
- showed
- showing
- shown
- Shows
- signals
- Signatures
- similar
- Similarly
- Simple
- simulations
- simultaneously
- single
- Size
- slightly
- Slowly
- small
- smooth
- So
- solid
- solution
- Space
- specific
- specificity
- Spectroscopy
- Spectrum
- split
- spread
- squares
- standard
- standards
- Stars
- State
- States
- statistically
- Still
- Strands
- structural
- structure
- studies
- Study
- substantial
- substantially
- such
- sufficient
- suggest
- suitability
- suitable
- suppressing
- surpasses
- T
- table
- TAG
- taken
- Target
- targeted
- targets
- Technical
- tens
- tested
- than
- that
- The
- their
- therefore
- These
- this
- thousand
- three
- three-dimensional
- threshold
- throughput
- Thus
- tighter
- tightly
- time
- to
- together
- top
- Total
- transfer
- transport
- Transport properties
- trapped
- trapping
- traps
- true
- tunes
- tuning
- two
- type
- types
- typical
- typically
- unchanged
- under
- unique
- uniquely
- until
- us
- USA
- use
- used
- useful
- uses
- using
- usually
- utilized
- value
- Values
- van
- variable
- variations
- various
- verified
- verify
- Versus
- via
- Video
- View
- visualization
- von
- W
- wang
- was
- washing
- watching
- we
- WELL
- were
- when
- whereas
- whether
- which
- Wilson
- with
- within
- without
- Work
- Worst
- would
- yellow
- zephyrnet